Method for analyzing latent genotoxic impurities of lamotrigine
A technique for detecting lamotrigine and a detection method, which is applied in the field of chemical analysis, and can solve problems such as insufficient detection sensitivity and inability to accurately quantify impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044]Chromatographic conditions
[0045]Instrument: High Performance Liquid Chromatograph Equipped with Mass Spectrometry Detector
[0046]Column: Waters Xbridge Shield, RP18, 150 × 4.6mm, 3.5μm
[0047]Mobile phase a: 0.1% formic acid aqueous solution
[0048]Mobile phase B: acetonitrile
[0049]Flow speed: 1.0ml / min
[0050]Inject volume: 5μL
[0051]Column temperature: 25 ° C
[0052]Running time: 30min
[0053] Time / min Mobile phase a / % Mobile phase B / % 08515 38515 152080 222080 22.18515 308515
[0054]The mass spectrometry detector parameters are as follows:
[0055]
[0056]
[0057]Diluent: dichloromethane
[0058]Derived reagent solution: It is weighed in 25 mg of waterless copiperazine to 25 mL volumetric flask, followed by dilution to the scale with dilution. (Derived reagent concentration: 1.0mg / ml)
[0059]White solution: Accurately remove 0.5 ml of dilution, derivatized reagent 0.5 ml to 1.5 ml into spots, mix well, sealed the cap, 2 hours under 40 ° C, and then removed to room temperature.
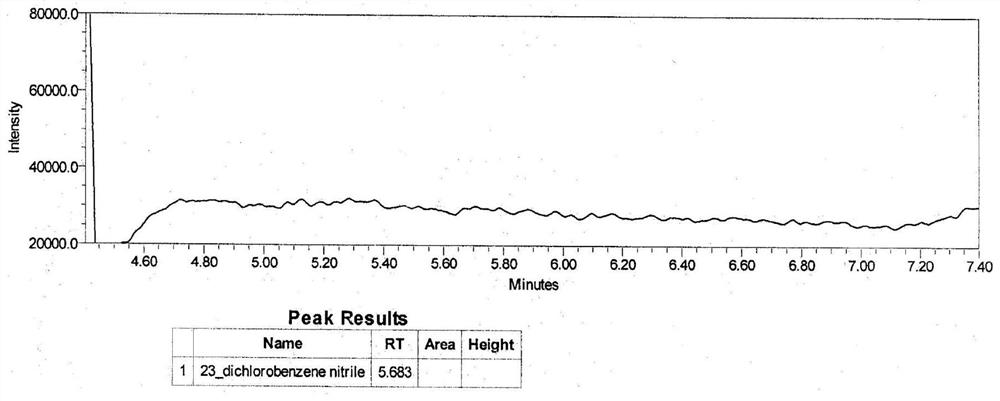
[0060]2,3-di...
Embodiment 2
[0067]Chromatographic conditions
[0068]Instrument: High Performance Liquid Chromatograph Equipped with Mass Spectrometry Detector
[0069]Column: ACE 3C18, 150mm * 4.6mm; 3.0μm
[0070]Mobile phase a: 0.1% formic acid aqueous solution
[0071]Mobile phase B: acetonitrile
[0072]Flow speed: 1.0ml / min
[0073]Inject volume: 5μL
[0074]Column temperature: 35 ° C
[0075]Running time: 40min
[0076] Time / min Mobile phase a / % Mobile phase B / % 08020 108020 252080 352080 35.18020 408020
[0077]The mass spectrometry detector parameters are as follows:
[0078]
[0079]Diluent: dichloromethane
[0080]Derived reagent solution: It is weighed in 25 mg N-methylpiperazine to 25 mL volumetric flask, followed by dilution to the scale with a dilution. (Derived reagent concentration: 1.0mg / ml)
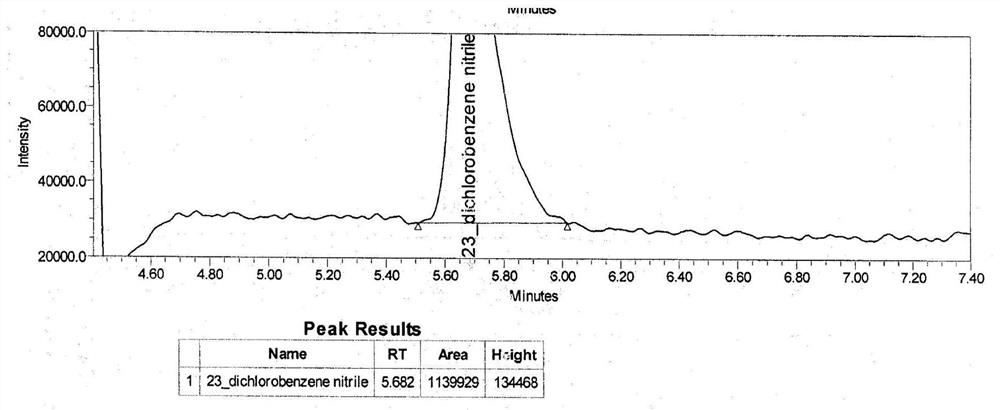
[0081]Blank solution, 2,3-dichlorobenzyl nitrile stock solution (2,3-dichlorobenzyl nitrile concentration: 42.8 ng / ml), sensitivity solution (2,3-dichloride) concentration: 6.4 Ng / mL), standard solution (2,3-dichlorodyl) concentr...
Embodiment 3
[0084]Chromatographic conditions
[0085]Instrument: High Performance Liquid Chromatograph Equipped with Mass Spectrometry Detector
[0086]Column: ACE 3C18, 150mm * 4.6mm; 3.0μm
[0087]Mobile phase a: 0.1% aqueous trifluoroacetic acid solution
[0088]Mobile phase B: methanol
[0089]Test wavelength: 210nm
[0090]Flow speed: 1.0ml / min
[0091]Inject volume: 5μL
[0092]Column temperature: 35 ° C
[0093]Running time: 30min
[0094] Time / min Mobile phase a / % Mobile phase B / % 08020 155545 182080 212080 258020 308020
[0095]The mass spectrometry detector parameters are as follows:
[0096]
[0097]
[0098]Diluent: dichloromethane
[0099]Derived reagent solution: It is weighed in 25 mg of waterless copiperazine to 25 mL volumetric flask, followed by dilution to the scale with dilution. (Derived reagent concentration: 1.0mg / ml)
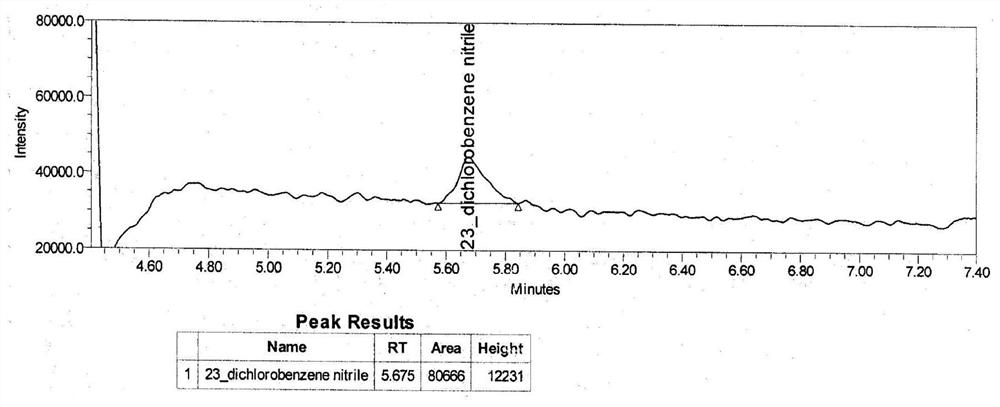
[0100]Blank solution, 2,3-dichlorobenzyl nitrile stock solution (2,3-dichlorobenzyl nitrile concentration: 42.8 ng / ml), sensitivity solution (2,3-dichloride) concentration: 6.4 Ng / ml), st...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Snr | aaaaa | aaaaa |
| Snr | aaaaa | aaaaa |
| Snr | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com