Method for continuously preparing bibenzyl by using microchannel reaction device
A technology of microchannel reaction and microchannel reactor, applied in chemical instruments and methods, chemical/physical/physicochemical reactors, condensation hydrocarbon production with dehydrocarbons, etc., can solve the need for metal catalysis, reaction process cycle Long and other problems, to achieve the effect of improving yield, good industrial scale-up potential, and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
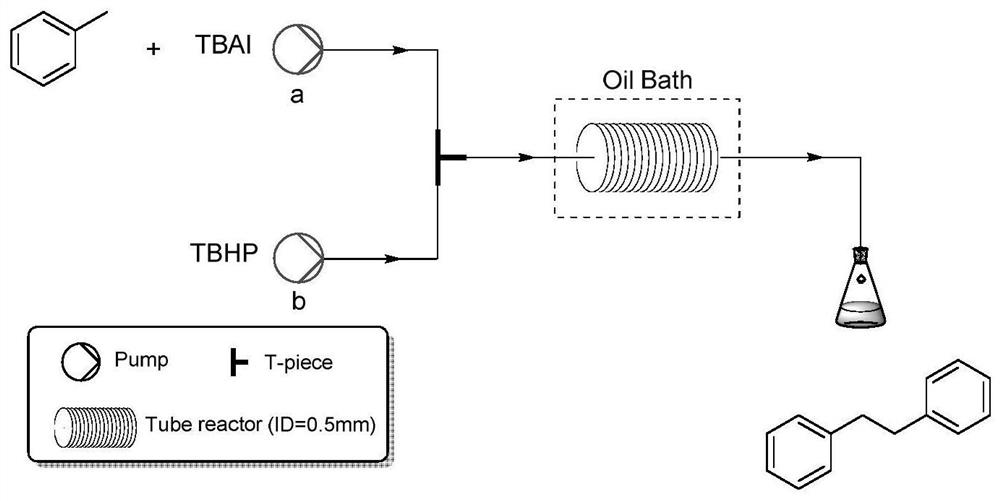
[0027] Dissolve 1 mmol (0.092 g) of toluene and 0.2 mmol (0.074 g) of tetrabutylammonium iodide in 5 mL of dimethyl sulfoxide and 5 mL of water to obtain a homogeneous solution A, which is added to syringe pump a; 2 mmol of TBHP (70% , 0.257g) (tert-butyl hydroperoxide) 5mL dimethyl sulfoxide and 5mL water to obtain a homogeneous solution B, which was added to syringe pump b; the injection flow rates of syringe pumps a and b were both 0.5ml / min; Microchannel reactor reaction volume V=10ml, reaction time 10min; Microchannel reactor internal diameter is 0.5mm; The yield of the product calculated by the method was 82%, and bibenzyl was obtained after separation by column chromatography.
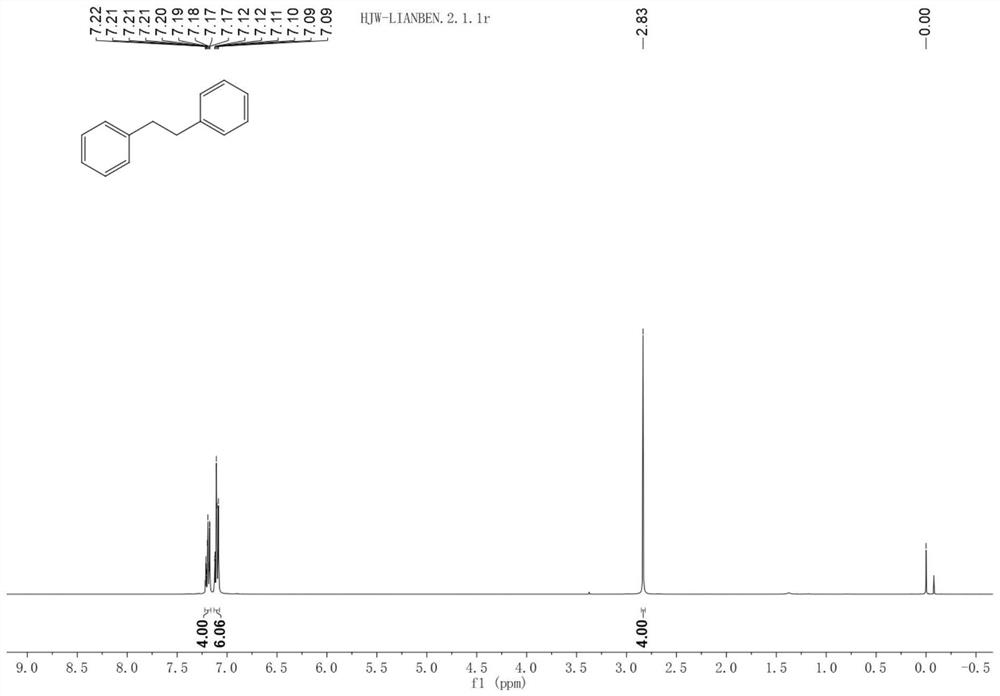
[0028] 1 H NMR (400MHz, Chloroform-d) δ7.22–7.16 (m, 4H), 7.11 (td, J=6.3, 1.5Hz, 6H), 2.83 (s, 4H).
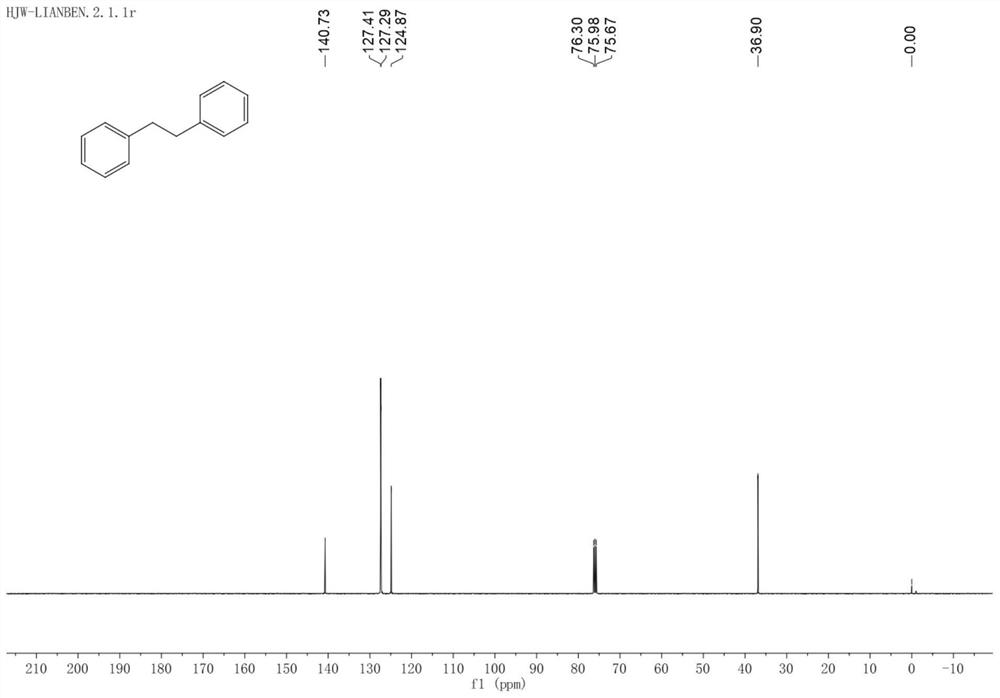
[0029] 13 C NMR (101MHz, Chloroform-d) δ140.73, 127.41, 127.29, 124.87, 36.90.
[0030] HRMS(TOF)m / z[M+H] + Calcd for C 14 h 15 183.1168 found 183.1165.
Embodiment 2
[0032] Dissolve 1 mmol (0.092 g) of toluene and 0.1 mmol (0.037 g) of tetrabutylammonium iodide in 5 mL of dimethyl sulfoxide and 5 mL of water to obtain a homogeneous solution A, which is added to syringe pump a; 2 mmol of TBHP (70% , 0.257g) (tert-butyl hydroperoxide) 5mL dimethyl sulfoxide and 5mL water to obtain a homogeneous solution B, which was added to syringe pump b; the injection flow rates of syringe pumps a and b were both 0.5ml / min; Microchannel reactor reaction volume V=10ml, reaction time 10min; Microchannel reactor internal diameter is 0.5mm; The yield of the product calculated by the method was 70%, and bibenzyl was obtained after separation by column chromatography.
Embodiment 3
[0034] Dissolve 1 mmol (0.092 g) of toluene and 0.3 mmol (0.074 g) of tetrabutylammonium iodide in 5 mL of dimethyl sulfoxide and 5 mL of water to obtain a homogeneous solution A, which is added to syringe pump a; 2 mmol of TBHP (70% , 0.257g) (tert-butyl hydroperoxide) 5mL dimethyl sulfoxide and 5mL water to obtain a homogeneous solution B, which was added to syringe pump b; the injection flow rates of syringe pumps a and b were both 0.5ml / min; Microchannel reactor reaction volume V=10ml, reaction time 10min; Microchannel reactor internal diameter is 0.5mm; The yield of the product calculated by the method was 77%, and bibenzyl was obtained after separation by column chromatography.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com