Application of allantoin in relieving side effects of glucocorticoid
A technology of glucocorticoids and side effects, applied in the field of compound drugs, can solve the problems of no research reports and achieve the effect of alleviating side effects and glucocorticoid-induced osteoporosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Example 1 MC3T3-E1 cell culture
[0058] After MC3T3-E1 cells were recovered from the liquid nitrogen tank, they were incubated in α-MEM medium (without Vc) containing 10% fetal bovine serum at 37°C, 5% CO 2 The MC3T3-E1 cells were continuously cultured in the incubator, and the medium was changed every 3 days. When the cells grew to cover about 90% of the bottom of the bottle, they were digested with 0.25% trypsin, centrifuged at 900rpm for 2min, and the cells were collected for the following procedures: experiment.
Embodiment 2
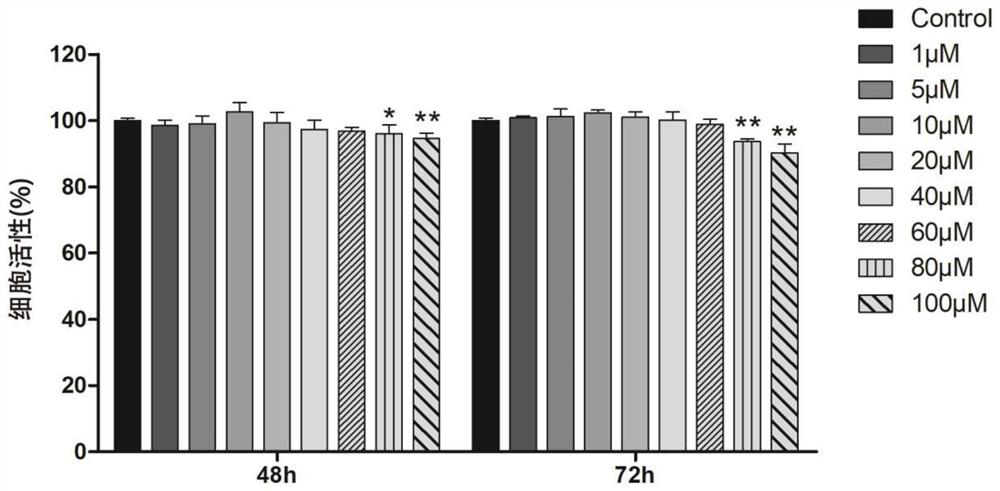
[0059] Example 2 The effect of allantoin on the activity of MC3T3-E1 cells detected by CCK-8 method
[0060] After the digested cells were prepared into a uniform single-cell suspension with α-MEM complete medium, the cell count was adjusted to a cell density of 3×10 3 cells / well; each well was inoculated with 100 μl of cell suspension in a 96-well plate, and cultured in a carbon dioxide incubator. After 24 hours of cell attachment, the cells were grouped as follows: ① 0 μM allantoin (Control group), ② 1 μM allantoin group, ③ 5 μM allantoin group, ④ 10 μM allantoin group, ⑤ 20 μM allantoin group, ⑥ 40 μM allantoin group group, ⑦60μM allantoin group, ⑧80μM allantoin group, ⑨100μM allantoin group. At 48h and 72h after administration, the cell proliferation was detected with a CCK-8 detection kit. Add 100 μl of CCK-8 working solution, put it into the incubator and continue to incubate for 2 hours. After the incubation time is reached, take out the 96-well cell culture plate, me...
Embodiment 3
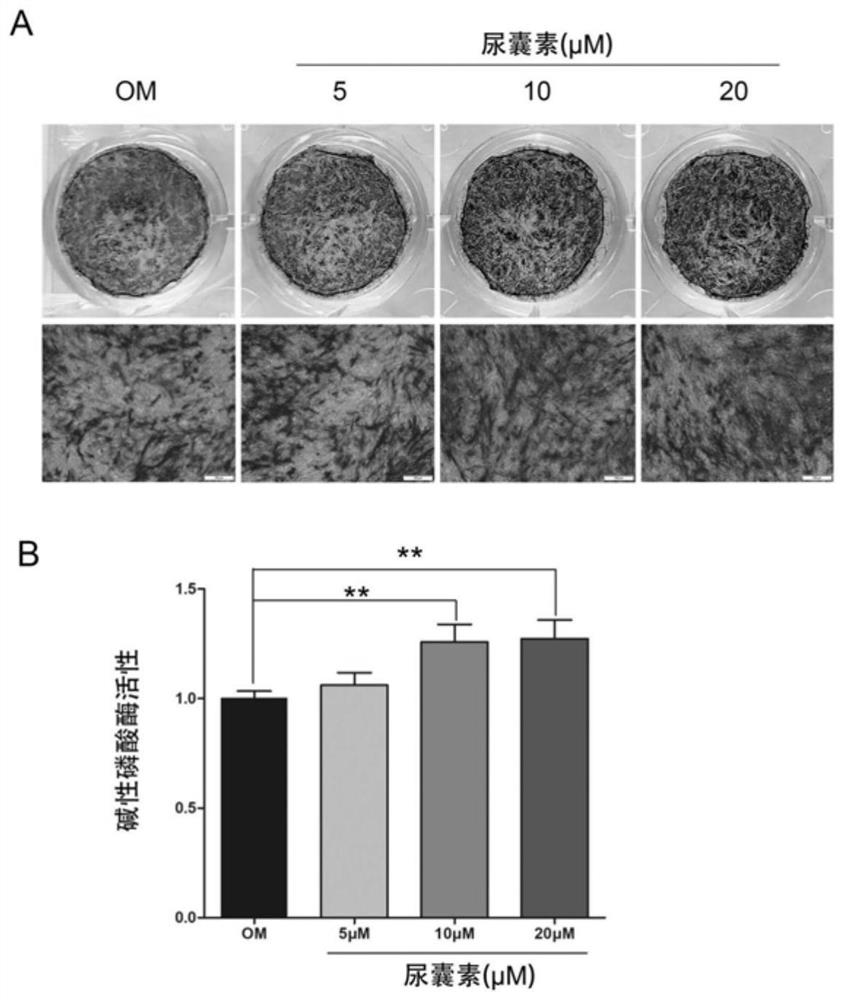
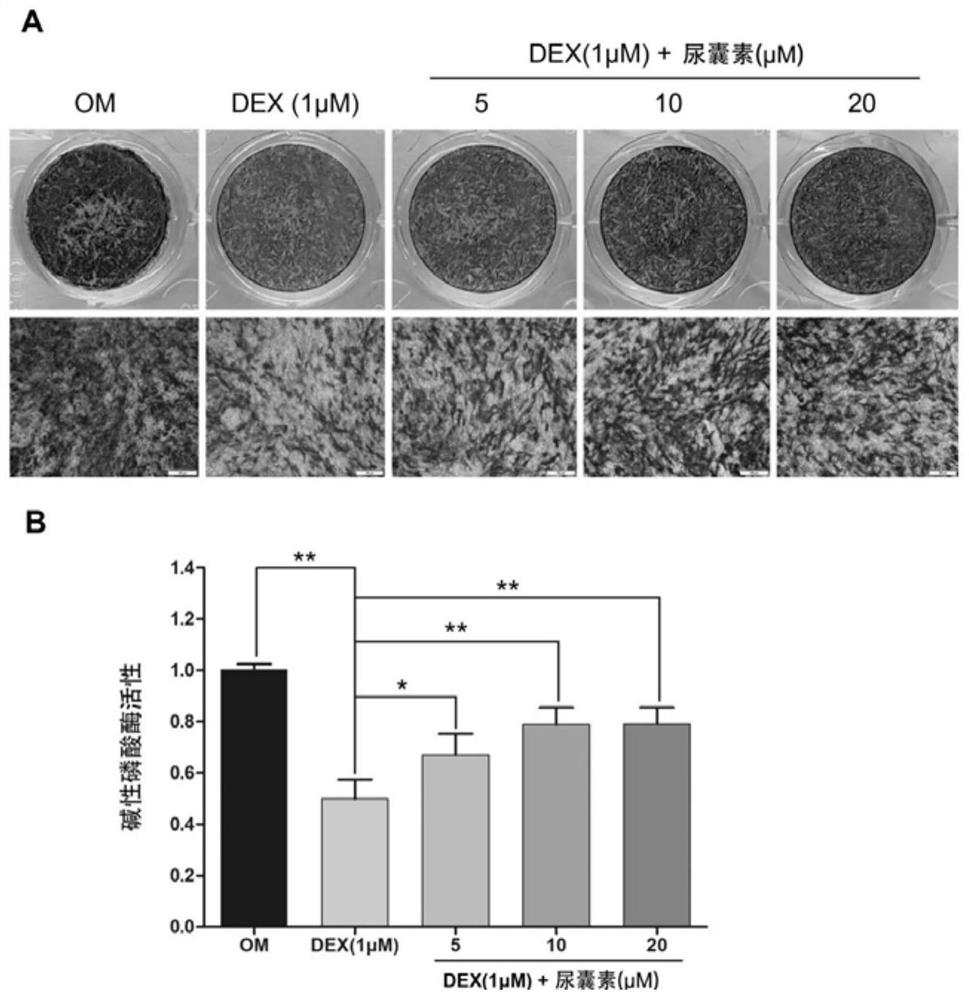
[0062] Example 3 Alkaline phosphatase staining and activity detection The effect of allantoin on the ALP activity of MC3T3-E1 cells
[0063] Alkaline phosphatase (ALP) is a marker glycoprotein in the early differentiation of osteoblasts, and the activity of ALP is closely related to the osteogenic differentiation of cells. In the early stages of osteogenic differentiation and functional maturity, the activity of ALP can be used as one of the indicators to evaluate bone formation and bone turnover. However, under the action of super-physiological amounts of glucocorticoids, the activity of alkaline phosphatase in the process of osteogenic differentiation in vitro will be significantly inhibited and affect the osteogenic differentiation ability of cells. In this experiment, alkaline phosphatase staining and quantitative detection of alkaline phosphatase activity were used to explore whether allantoin could promote the activity of alkaline phosphatase in MC3T3-E1 cells.
[0064]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com