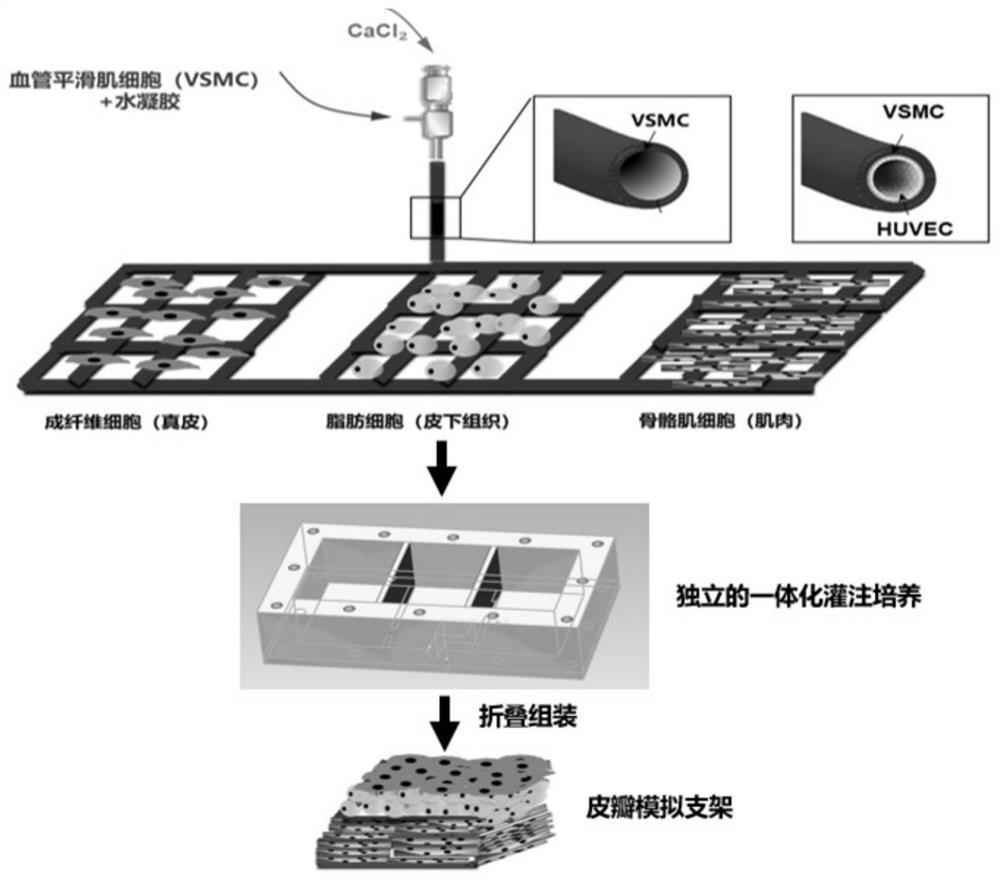
Making method of three-dimensional vascularized musculocutaneous flap based on biological 3D printing
A 3D printing and manufacturing method technology, applied in biochemical equipment and methods, vascular endothelial cells, microorganisms, etc., can solve problems such as the difficulty in realizing the independent culture and differentiation of different cell components, and the difficulty in realizing the integration of blood flow and perfusion of different cell components. , to achieve the effect of high biocompatibility and improved survival rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
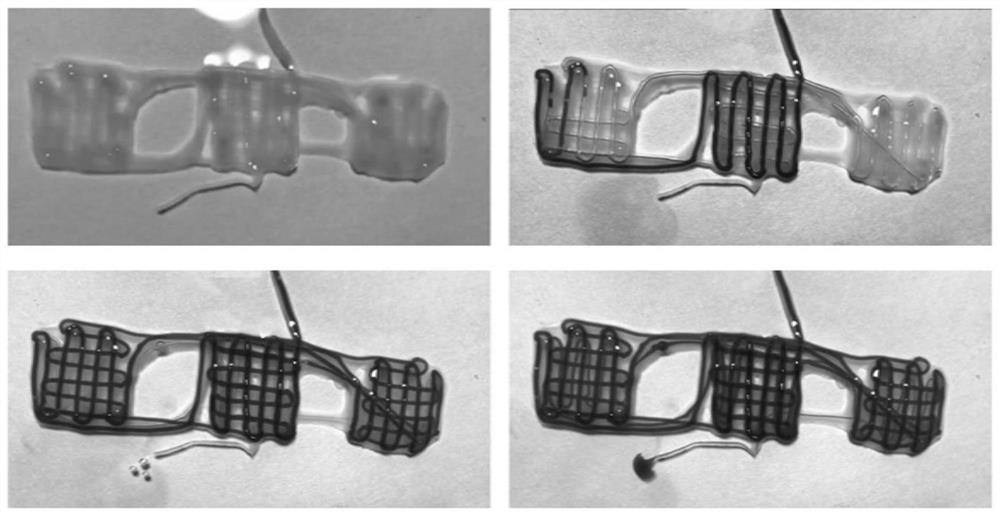
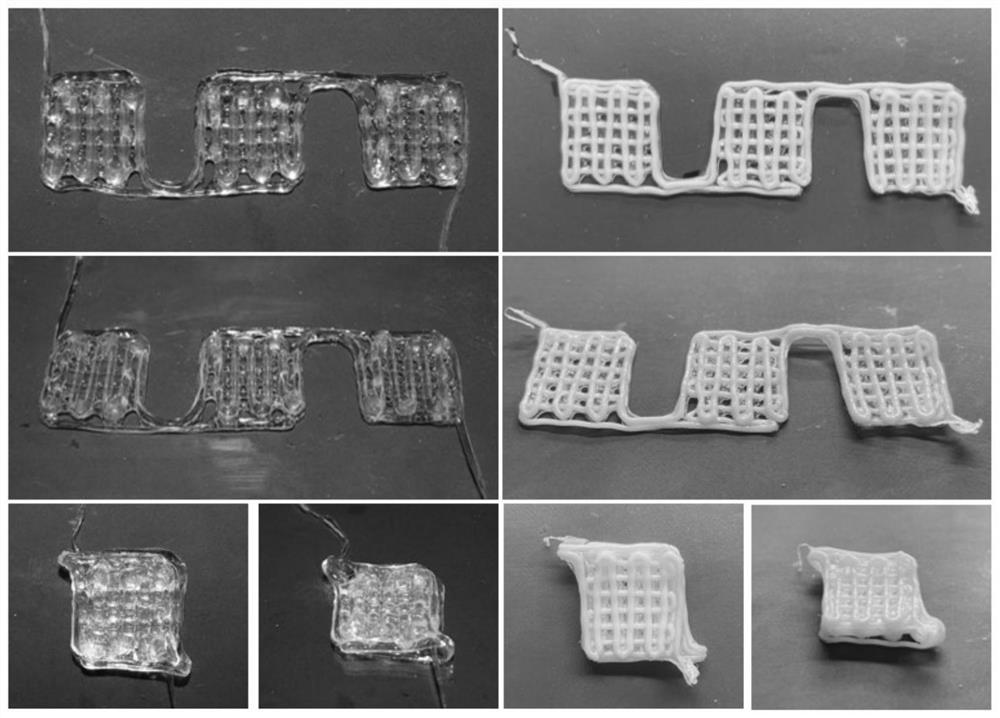
[0030] A method for making a three-dimensional vascularized myocutaneous flap based on biological 3D printing, the specific steps are as follows: (1) according to the mass volume ratio of 5% GelMA (methacryloyl gelatin), 2% sodium alginate, 10% PEGDA ( Polyethylene glycol diacrylate) and 0.05% LAP were dissolved in 25 mM 4-hydroxyethylpiperazineethanesulfonic acid buffer solution (HEPES). Add vascular smooth muscle cells to make the cell concentration 10^7 cells / mL. Ink was stored at 37°C until use. Prepare 50mM CaCl 2 solution as an ionic crosslinker. Three-dimensional tubular structures are printed using a two-channel coaxial system. Inner layer is 50mM CaCl 2 Solution, using a 20mL syringe, the extrusion speed is 0.1mm / min. The outer layer is bio-ink, using a 5mL syringe with an extrusion speed of 0.56mm / min. The bioprinted constructs were first treated with CaCl 2 solution cross-linked and then exposed to blue light (395nm, 8mW / cm 2 Power) 5min for further cross-li...
Embodiment 2
[0034] A method for making a three-dimensional vascularized myocutaneous flap based on biological 3D printing, the specific steps are as follows: (1) 7% GelMA (methacrylated gelatin), 1% sodium alginate, 15% PEGDA (polyethylene glycol Diacrylate) and 0.1% LAP were dissolved in HEPES (25mM). Add vascular smooth muscle cells to make the cell concentration 10^7 cells / mL. Ink was stored at 37°C until use. Prepare 30mM CaCl 2 solution as an ionic crosslinker. Three-dimensional tubular structures are printed using a two-channel coaxial system. The inner layer is 30mM CaCl 2 Solution, using a 20mL syringe, the extrusion speed is 0.15mm / min. The outer layer is bio-ink, using a 5mL syringe with an extrusion speed of 0.84mm / min. The bioprinted constructs were first treated with CaCl 2 solution cross-linked and then exposed to blue light (395nm, 8mW / cm 2Power) 7min for further cross-linking. Connect the stent through a 26G indwelling needle, and inject 0.5 ml of vascular endothe...
Embodiment 3
[0038] A method for making a three-dimensional vascularized myocutaneous flap based on biological 3D printing, the specific steps are as follows: (1) 5% GelMA (methacrylated gelatin), 3% sodium alginate, 10% PEGDA (polyethylene glycol Diacrylate) and 0.05% LAP were dissolved in HEPES (25 mM). Add vascular smooth muscle cells to make the cell concentration 10^7 cells / mL. Ink was stored at 37°C until use. Prepare 75mM CaCl 2 solution as an ionic crosslinker. Three-dimensional tubular structures are printed using a two-channel coaxial system. The inner layer is 75mM CaCl 2 Solution, using a 20mL syringe, the extrusion speed is 0.1mm / min. The outer layer is bio-ink, using a 5mL syringe with an extrusion speed of 0.56mm / min. The bioprinted constructs were first treated with CaCl 2 solution cross-linked and then exposed to blue light (395nm, 8mW / cm 2 Power) 7min for further cross-linking. Connect the stent through a 26G indwelling needle, and inject 0.8 ml of vascular endot...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com