Method for preparing D-p-methylsulfonyl phenyl serine ethyl ester through immobilized enzyme catalysis
A technology of thiamphenylphenylserine ethyl ester and immobilized enzymes, applied to biochemical equipment and methods, and enzymes immobilized on or in inorganic carriers, can solve the problems of complicated synthesis process and easy inactivation of enzymes, etc. Achieve the effects of high selectivity, improved utilization, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
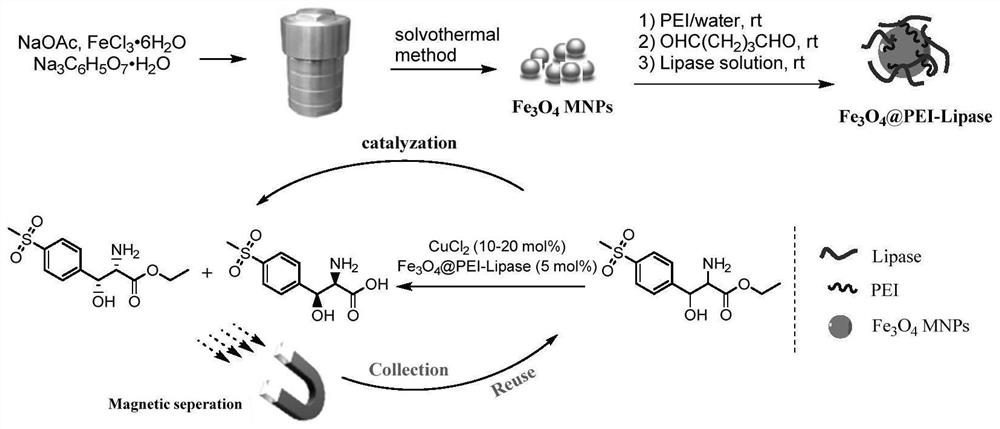
[0034] 1) Superparamagnetic Fe 3 o 4 Enzymes immobilized on nanoparticles (Fe 3 o 4 Preparation of @PEI-Lipase)
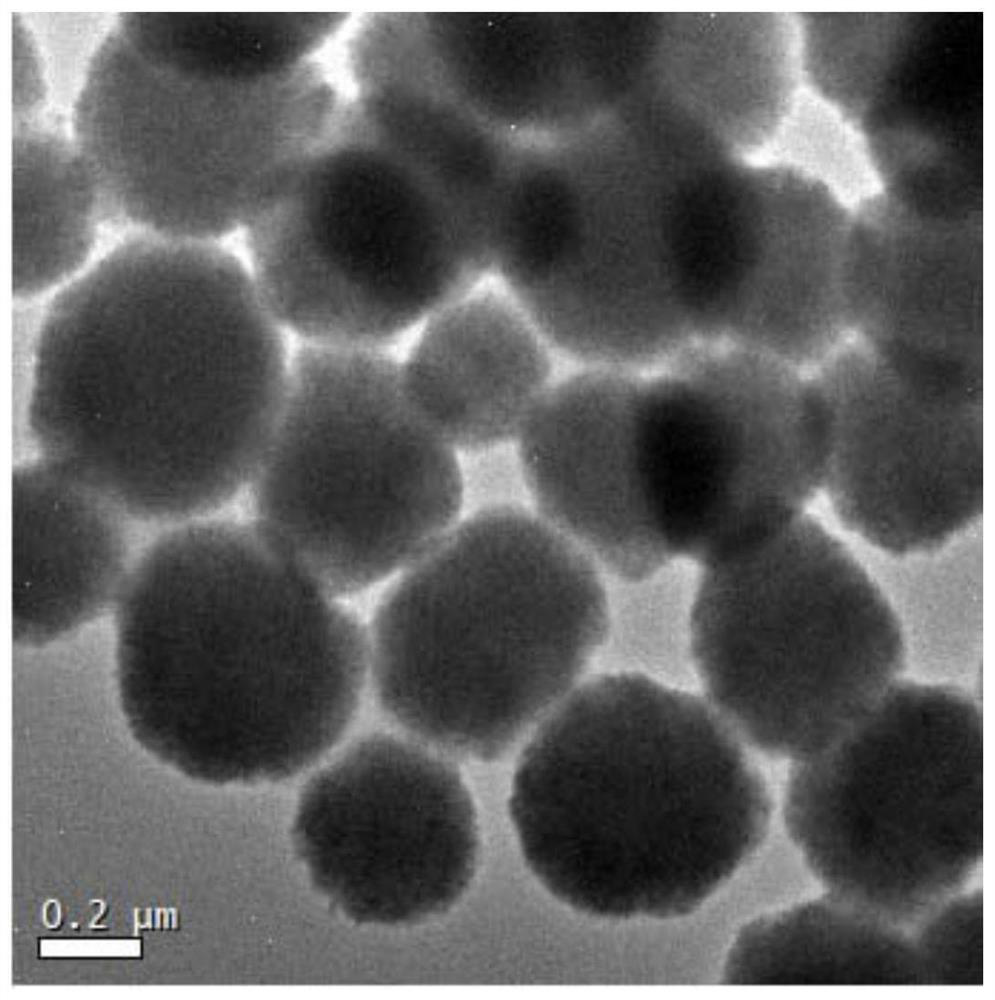
[0035] 6.48g of ferric chloride hexahydrate, 4.704g of sodium citrate and 9.84g of sodium acetate were dissolved in 400mL of ethylene glycol, and after the mixture was dispersed evenly by ultrasonic waves, it was distributed into ten 100mL polytetrafluoroethylene autoclaves. React at 140°C for 12 hours. After cooling down to room temperature, the crude product is separated by a magnet and washed with water and ethanol for 3-5 times (to neutrality). Vacuum dried for later use.
[0036] Above-mentioned magnetic nanoparticles 5g and PEI (M w =10000) 25g was dissolved in 60mL of distilled water, and the reaction solution was vigorously stirred for 24 hours, then magnetically separated. The solid was washed 3-4 times with distilled water (20mL) and dried in vacuum for subsequent use (marked as Fe 3 o 4 @PEI).
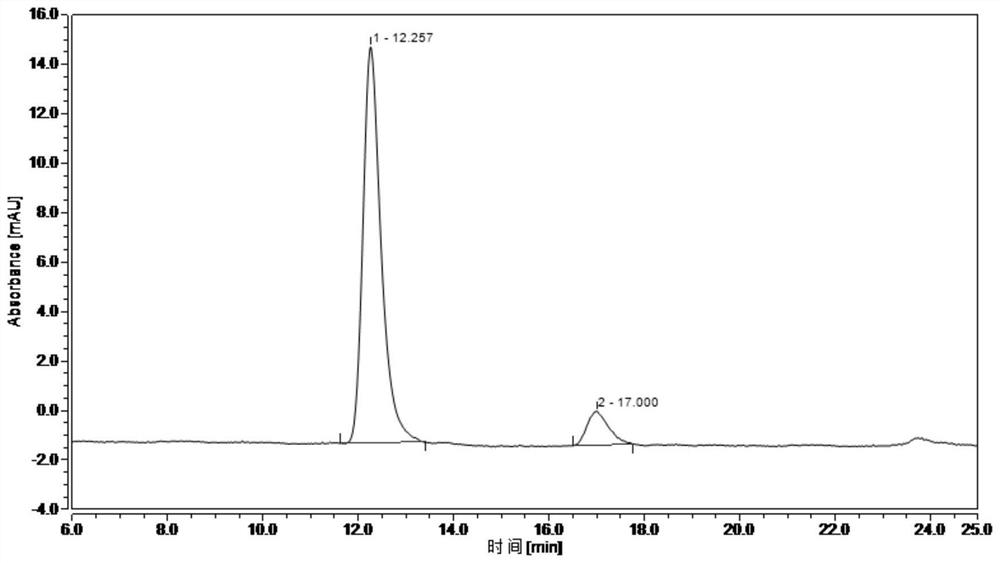
[0037] The aforementioned PEI-activated magnetic n...
Embodiment 2
[0046] 1) Superparamagnetic Fe 3 o 4 Enzymes immobilized on nanoparticles (Fe 3 o 4 Preparation of @PEI-Lipase)
[0047] 6.48g of ferric chloride hexahydrate, 4.704g of sodium citrate and 9.84g of sodium acetate were dissolved in 400mL of ethylene glycol. After the mixture was dispersed evenly by ultrasonic, it was distributed into ten 100mL polytetrafluoroethylene autoclaves. React at 200°C for 6 hours. After cooling down to room temperature, the crude product is separated by a magnet and washed with water and ethanol for 3-5 times (to neutrality). Vacuum dried for later use.
[0048] 5 g of the above-mentioned magnetic nanoparticles and 25 g of PEI were dissolved in 60 mL of distilled water, and the reaction was vigorously stirred for 24 hours before magnetic separation. The solid was washed 3-4 times with distilled water (20mL) and dried in vacuum for subsequent use (marked as Fe 3 o 4 @PEI).
[0049] Activation and the immobilization of enzyme are the same as embod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com