Photocatalytic material capable of being used for reducing nitrogen to produce ammonia as well as preparation method and application of photocatalytic material
A photocatalytic material, nitrogen technology, applied in the preparation/separation of ammonia, chemical instruments and methods, ammonia compounds, etc., can solve the problems of rarely having absorption properties in the infrared spectral region, low solar energy utilization, and high proportion of precious metals, reaching Excellent photocatalytic reduction of nitrogen to produce ammonia, high solar energy utilization, and low proportion of precious metals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
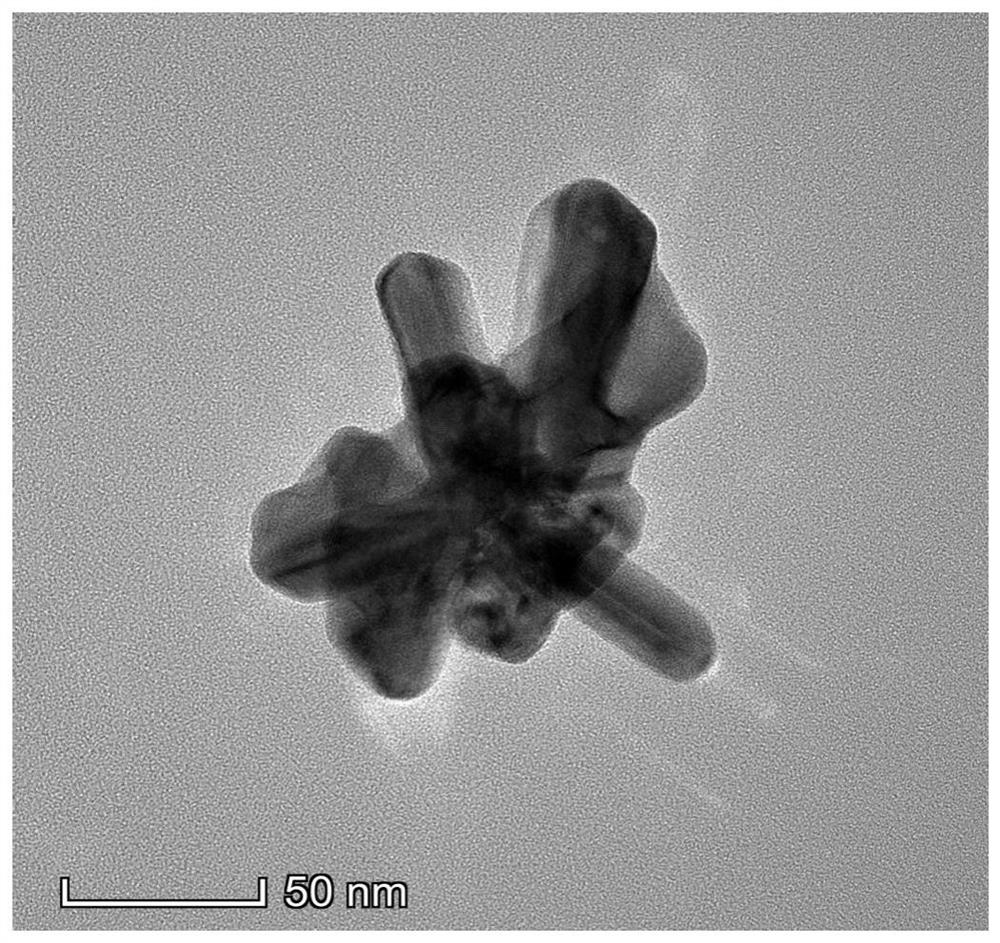
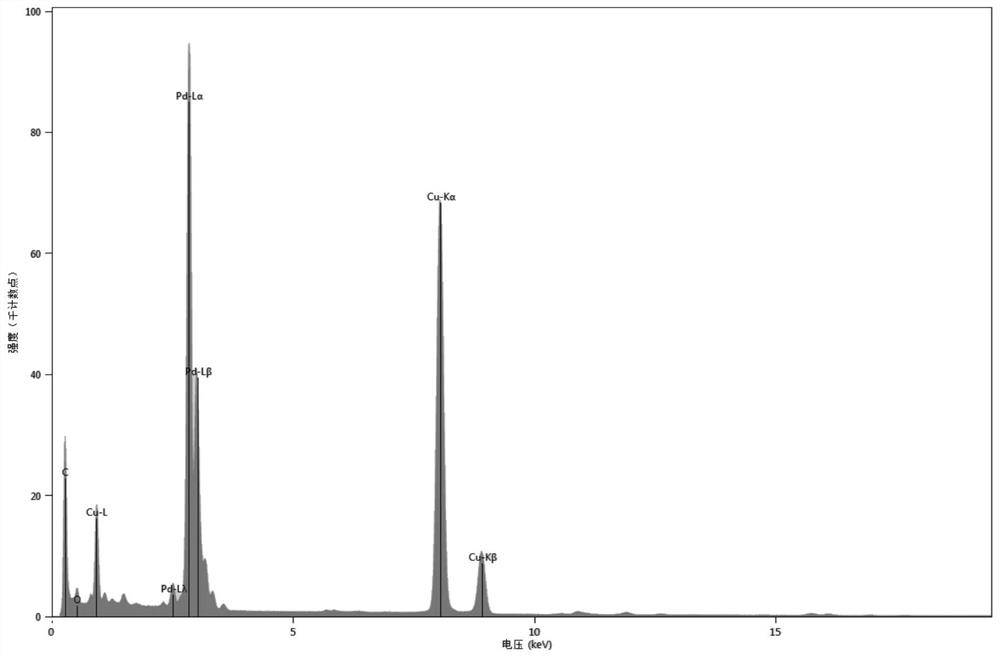
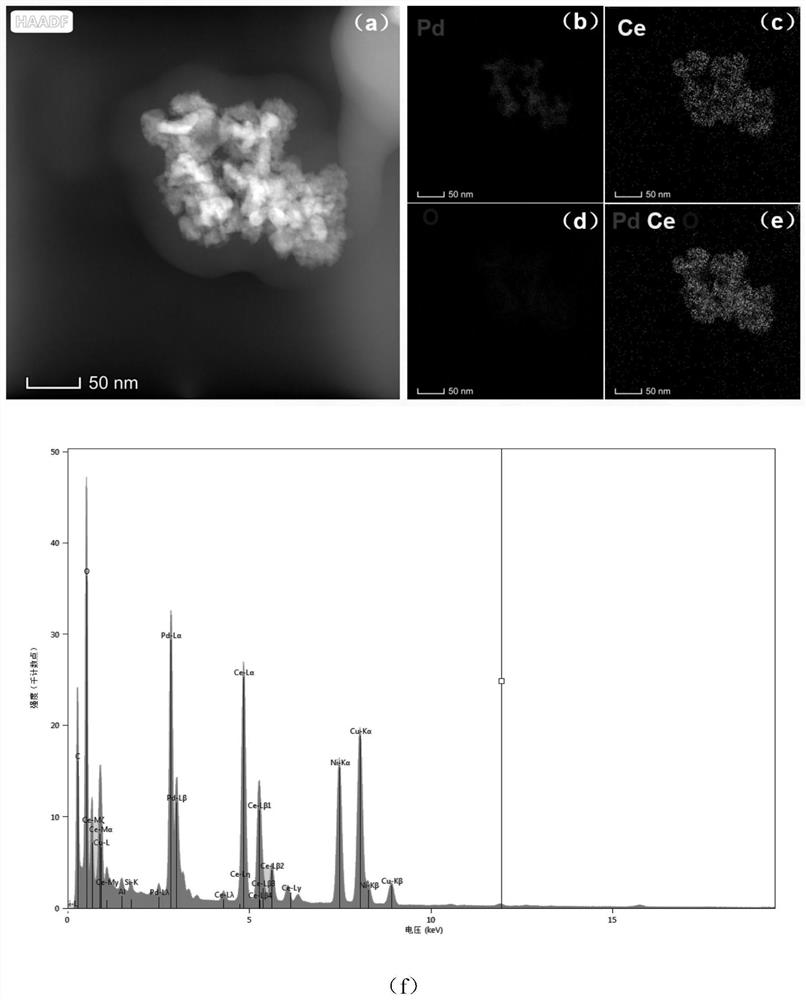
[0096] Pd@CeO for Photocatalytic Reduction of Nitrogen to Ammonia 2 Nanomaterials, which are composed of flower-shaped nano-palladium particles and CeO grown on the outer layer 2 layer shell, in which the atomic number ratio of Pd:Ce is about 50:50. The preparation method is as follows:
[0097] (1) Preparation of Pd seeds: prepare 5mL of 0.5mM PdCl 2 aqueous solution and 5mL 2mM CTAB aqueous solution, and inert gas was passed for 30 minutes to remove the dissolved oxygen in the two solutions, and 5mL 0.5mM PdCl after removing the dissolved oxygen 2 Mix the aqueous solution with 5mL 2mM CTAB aqueous solution, and then inject 250μL 15mM NaBH into the mixed solution 4 aqueous solution. A Pd seed solution was prepared.
[0098] (2) Preparation of flower-shaped nano-palladium particles: prepare 610mL of 0.25mM PdCl in No. 1 bottle 2 Aqueous solution, weigh 365mg CTAB in the No. 2 bottle, and then take the prepared PdCl in the No. 1 bottle 2 Add 10mL of the aqueous solution ...
Embodiment 2
[0104] Pd@CeO for Photocatalytic Reduction of Nitrogen to Ammonia 2 Nanomaterials, which are composed of flower-shaped nano-palladium particles and CeO grown on the outer layer 2 layer shell, in which the atomic number ratio of Pd:Ce is about 40:60. The preparation method is as follows:
[0105] (1) Preparation of Pd seeds: prepare 5mL of 0.5mM PdCl 2 aqueous solution and 5mL 2mM CTAB aqueous solution, and inert gas was passed for 30 minutes to remove the dissolved oxygen in the two solutions, and 5mL 0.5mM PdCl after removing the dissolved oxygen 2 Mix the aqueous solution with 5mL 2mM CTAB aqueous solution, and then inject 300μL 15mM NaBH into the mixed solution 4 aqueous solution. A Pd seed solution was prepared.
[0106] (2) Preparation of flower-shaped nano-palladium particles: prepare 610mL of 0.25mM PdCl in No. 1 bottle 2 Aqueous solution, weigh 365mg CTAB in the No. 2 bottle, and then take the prepared PdCl in the No. 1 bottle 2 Add 10 mL of the aqueous solution...
Embodiment 3
[0110] Preparation of Pd@CeO for photocatalytic reduction of nitrogen to produce ammonia in a similar manner to Example 1 2 Nanomaterials, in which flower-shaped nano-palladium particles grow a layer of CeO with a thickness of about 35 nm 2 , the atomic number ratio of Pd and Ce is about 35:65.
[0111]Using the same method as in Example 1, the photocatalytic material Pd@CeO prepared above 2 The photocatalytic ammonia production stability test was carried out, and the efficiency of the photocatalytic material to synthesize ammonia was about 76 μmol g -1 h -1 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com