Method for detecting lincomycin impurity E in lincomycin hydrochloride injection by high performance liquid chromatography-evaporative light method
A high-performance liquid chromatography, lincomycin hydrochloride technology, which is applied in measuring devices, instruments, scientific instruments, etc., can solve the problems of inability to accurately identify and detect impurity E, quantitative detection, and inability to characterize lincomycin impurity E.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The detection method of the present invention is used to detect lincomycin impurity E in lincomycin hydrochloride injection, wherein the chromatographic conditions for detection are evaporative light scattering detector drift tube temperature 50°C; carrier gas flow rate is 1.5L / min; The temperature is 30°C; the flow rate is 0.9ml / min.
[0038] The detection data of each concentration gradient of lincomycin impurity E standard substance are shown in Table 1:
[0039] Table 1 Detection data of each concentration gradient of lincomycin impurity E standard
[0040]
[0041]
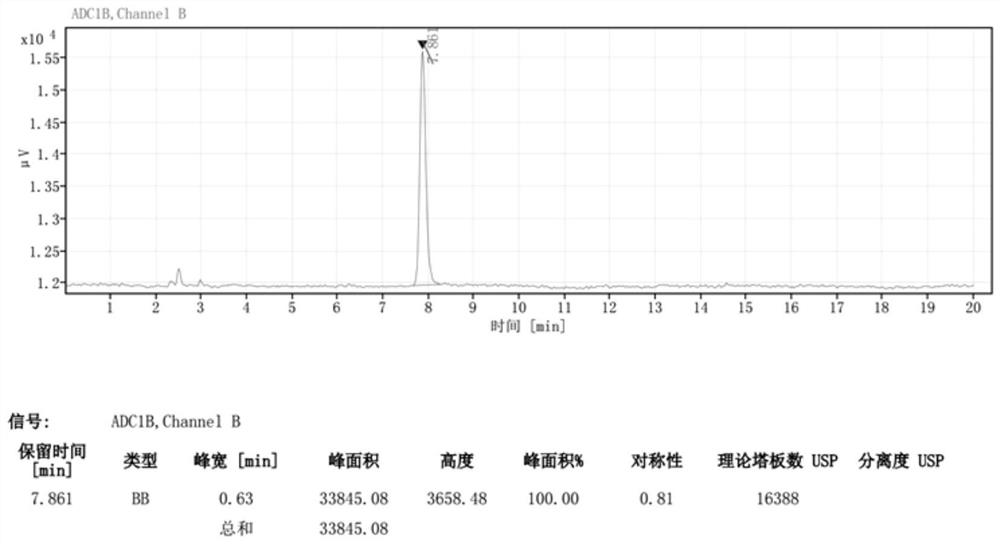
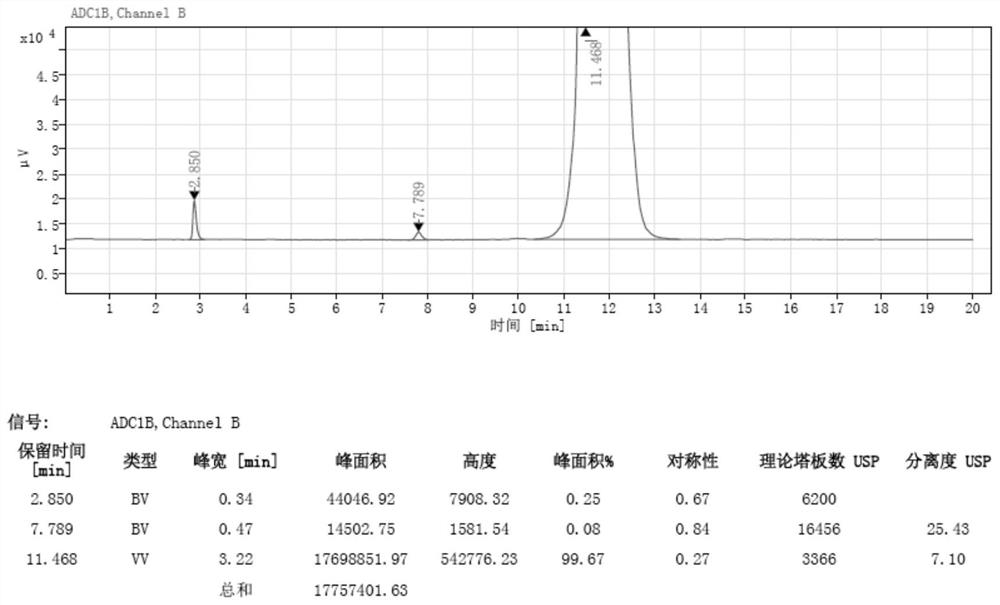
[0042] Wherein, the chromatogram of lincomycin impurity E standard substance 2 is as follows figure 1 As shown, the chromatograms of the first group of sample solutions to be tested are as follows figure 2 shown.
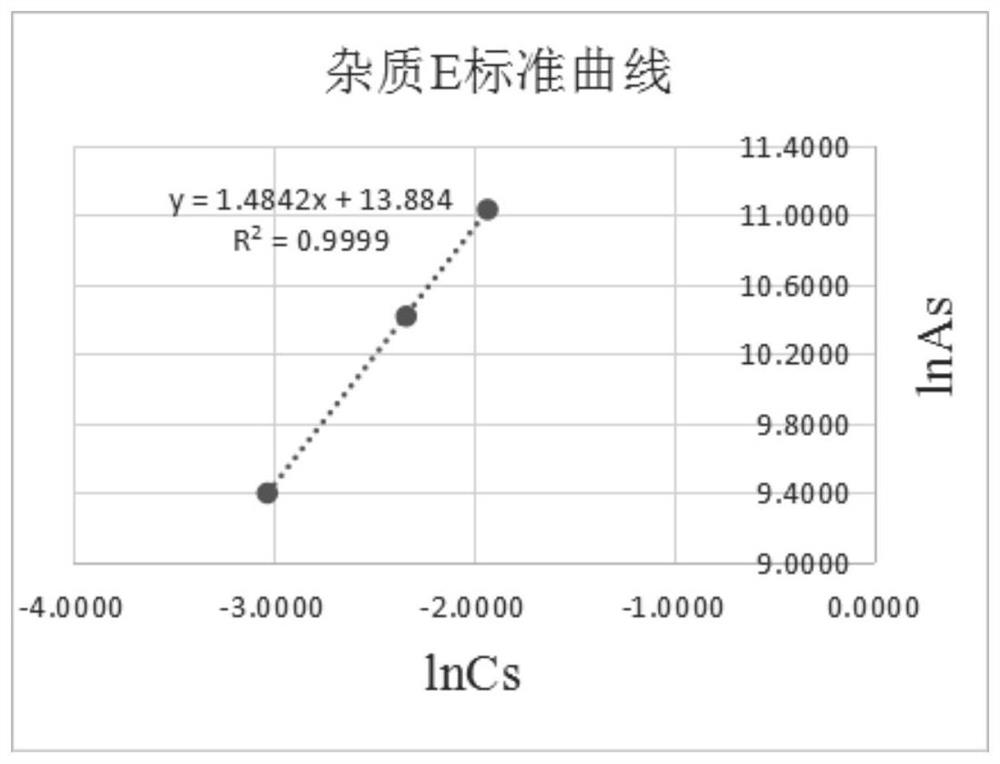
[0043] Draw the standard curve of lincomycin impurity E standard substance according to the detection data such as the concentration of above-mentioned standard substance and peak ...
Embodiment 2
[0052] The detection method of the present invention is used to detect lincomycin impurity E in lincomycin hydrochloride injection, wherein the chromatographic conditions for detection are evaporative light scattering detector drift tube temperature 60°C; carrier gas flow rate is 2.2L / min; The temperature is 35°C; the flow rate is 1.2ml / min.
[0053] In the second group of sample solution to be tested, the chromatogram of lincomycin impurity E is as follows Figure 4 shown.
[0054] Using the chromatographic conditions in this example, the detected lincomycin impurity E had a peak eluting time of 7.794 minutes and a resolution of 25.58. This method can still qualitatively and quantitatively detect lincomycin impurity E.
Embodiment 3
[0056] The lincomycin impurity E standard substance and the third group of sample solutions to be tested were detected by using the high-performance liquid chromatography ultraviolet detection method recorded in the prior art "Chinese Pharmacopoeia".
[0057] Wherein, the chromatographic conditions for the ultraviolet detection of the high-performance liquid chromatograph are as follows: the detection wavelength is 214nm; the column temperature is 30°C; and the flow rate is 0.5ml / min.
[0058] The chromatogram of the lincomycin impurity E standard substance detected by high performance liquid chromatography is as follows Figure 5 As shown, the chromatograms of the third group of sample solutions to be tested are as follows Figure 6 shown.
[0059] In the present embodiment, the chromatogram of lincomycin impurity E standard Figure 5 It shows that its peak time is 9.581min, which leads to the chromatogram of the third group of sample solutions to be tested. Figure 6 Amon...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com