Macrolactam compound FW05328-d and efficient fermentation method thereof
A technology of fw05328-d and macrocyclic lactam, which is applied in the field of structural analogues of macrocyclic lactam compound FW05328-1, can solve problems such as unstable properties, difficulty in large-scale preparation of FW05328-1, and achieve good medical value Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Shake flask fermentation of embodiment 1 compound FW05328-d
[0043] The preserved Micromonospora marine strain Micromonospora sp.FIM-MA181224 was cultured on a high asparagine solid medium at 35°C for 15 days to obtain a fresh slant of Micromonospora marine micromonospora sp.FIM-MA181224.
[0044] Inoculate the fresh slant excavated pieces (0.5cm×0.5cm) of Micromonospora marine Micromonospora sp.FIM-MA181224 in the shake flask seed medium, and cultivate them at 30°C and 240r / min for 72h to obtain the shake flask seed culture solution. The shake flask seeds were inoculated into the shake flask fermentation medium according to the inoculum amount of 4%, cultured at 32°C and 260r / min for 5 days, and then placed in the bottle, and the content of FW05328-d in the fermentation broth was determined by HPLC.
[0045] Seed medium preparation method: soluble starch 15g, glucose 5g, peptone 5g, yeast powder 5g, (NH 4 ) 2 SO 4 0.5g, K 2 HPO 4 ·3H 2 O 0.5g, CaCO 3 2g, sea s...
Embodiment 2
[0048] Purification and preparation of embodiment 2 compound FW05328-d
[0049] Take 10L of the fermentation product of Micromonospora sp. FIM-MA181224 in the above-mentioned Example 1 and centrifuge at 4500rpm to obtain mycelium, which is extracted with 1.5 times the volume of acetone (1:1, V:V) , the extract was concentrated under reduced pressure at less than 40°C to remove acetone to obtain an extract (5g). After dissolving the extract with appropriate distilled water, it was adsorbed with HP20 macroporous resin, eluted with 3 times the column volume of deionized water, and then used Gradient elution of 30%-75% alcohol concentration, HPLC detection and tracking, respectively pooled fractions containing component FW05328-d.
[0050] Then choose reverse C18 packing, prepacked column with diameter-to-height ratio of 1:3, absorb the above-mentioned substances on medium-pressure reverse C18 column, use methanol water 40%-80% gradient elution, HPLC detection and tracking, respec...
Embodiment 3
[0052] The structure identification of embodiment 3 compound FW05328-d
[0053] The compound FW05328-d obtained above is a light yellow amorphous powder. The structure of the target compound FW05328-d was identified by combining the data of mass spectrum, ultraviolet spectrum and nuclear magnetic resonance.
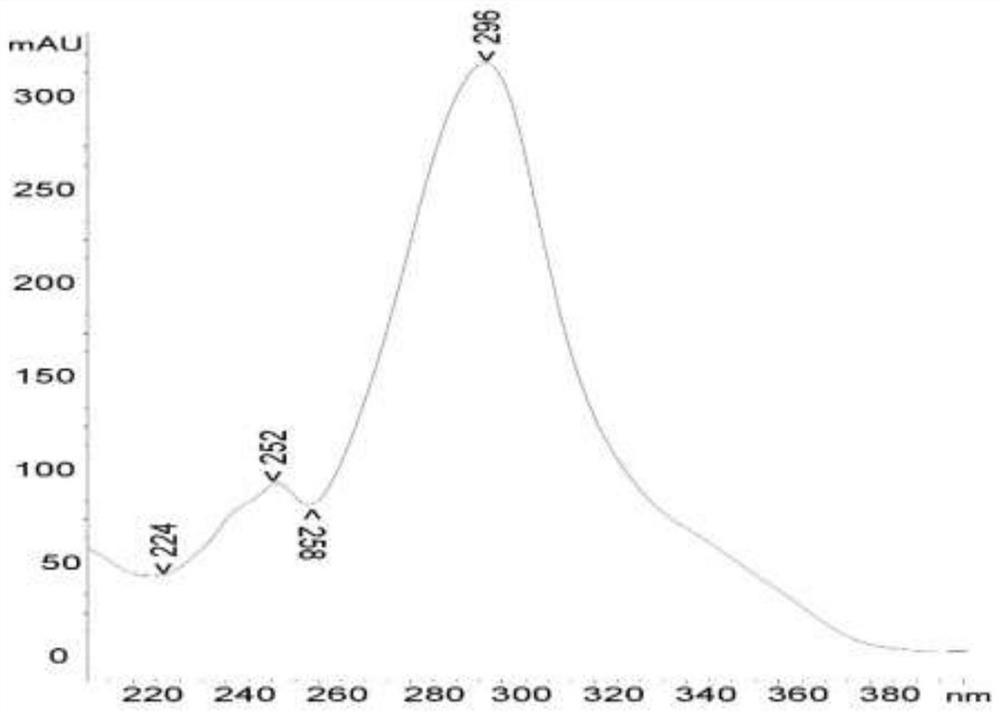
[0054] Such as figure 1 The ultraviolet absorption spectrum shown, UV(MeOH), λmax: 294 nm.
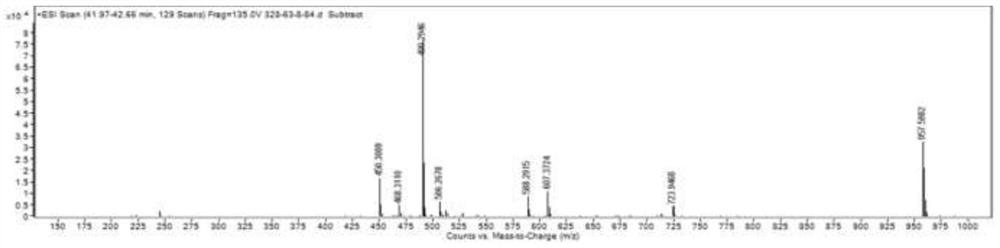
[0055] Such as figure 2 The shown HR-TOF-MS spectrum, HR-ESI-MS (m / z 490.2946[M+Na]+; molecular formula is C29H41NO4; calculated unsaturation is 10.
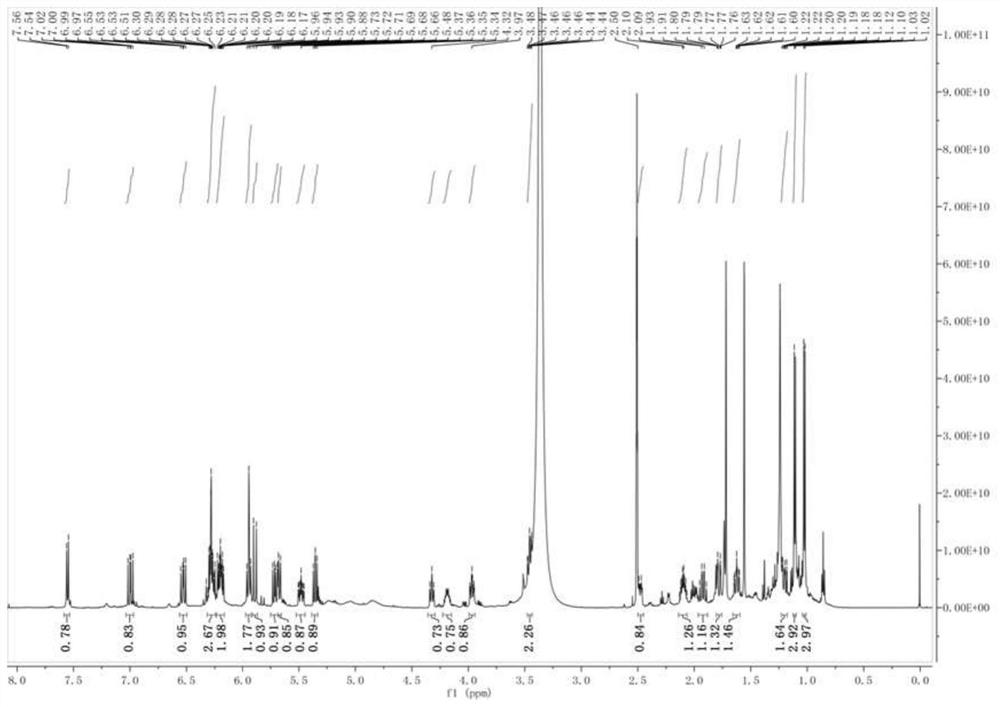
[0056] Such as image 3 shown 1 H-NMR (600MHz, in DMSO-d6) spectrum, it can be seen that the high field area shows two sets of double peak methyl hydrogen signals [δ1.03(3H,d,J=6.6Hz) and δ1.11(3H,d,J =7.0Hz)] and 2 sets of unimodal methyl hydrogen signals [δ1.56(3H,s) and δ1.72(3H,s)], there are multiple sets of ene hydrogen proton signals and 1 set of active hydrogen protons in the low field area Signal δ 7.55 (IH, d, J = ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com