Escherichia coli strain and method for biosynthesizing pyranocoumarin and furocoumarin by using escherichia coli strain
A kind of Escherichia coli, biosynthesis technology, applied in the field of synthetic biology, can solve the problems of complex extraction and purification process, high cost, low content, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034]Example 1 Plasmid construction of synthetic pathways for quincetin, dihydroeosanthin, Japanese procurerol and lomatin
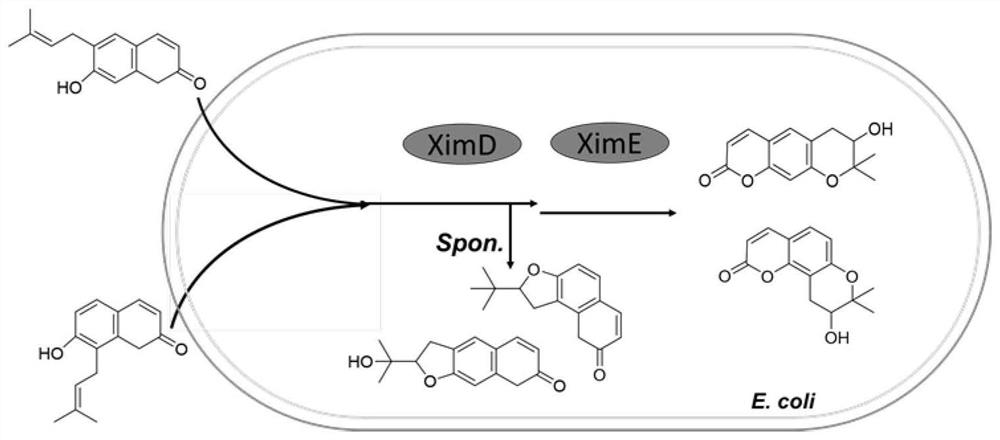
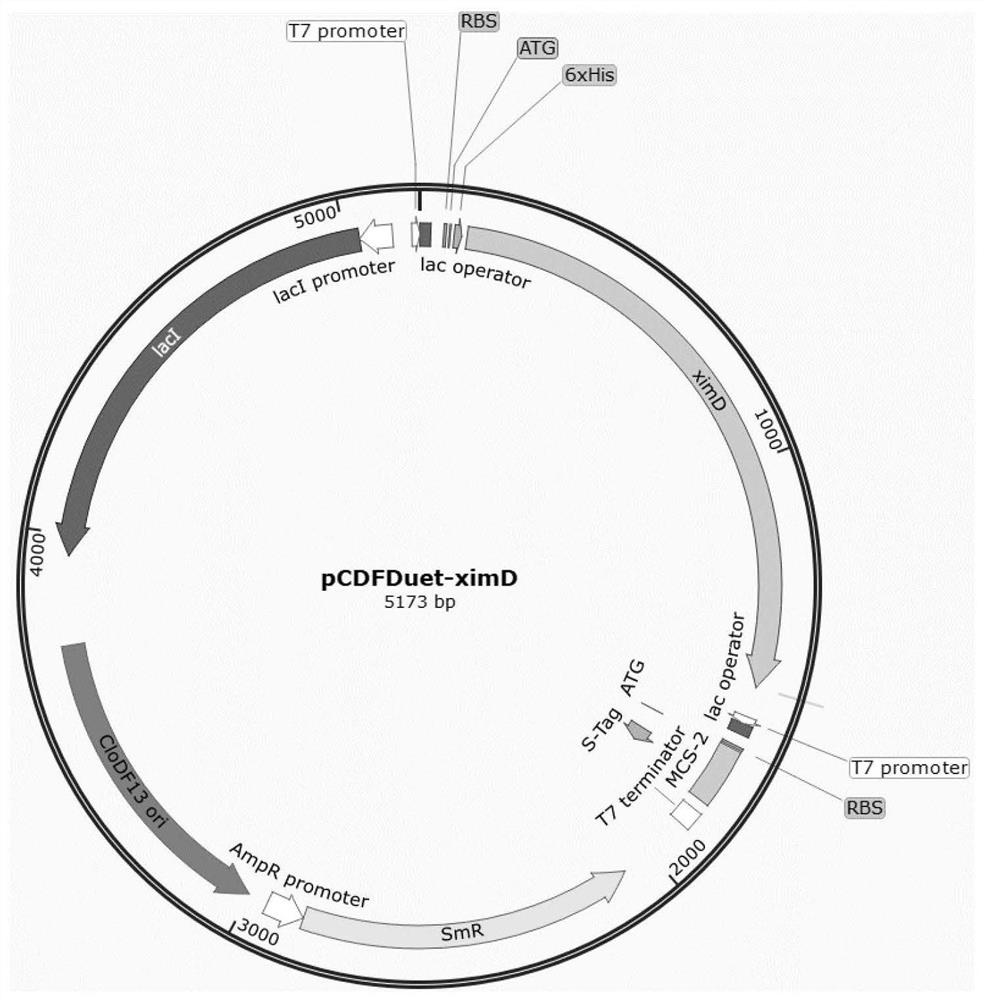
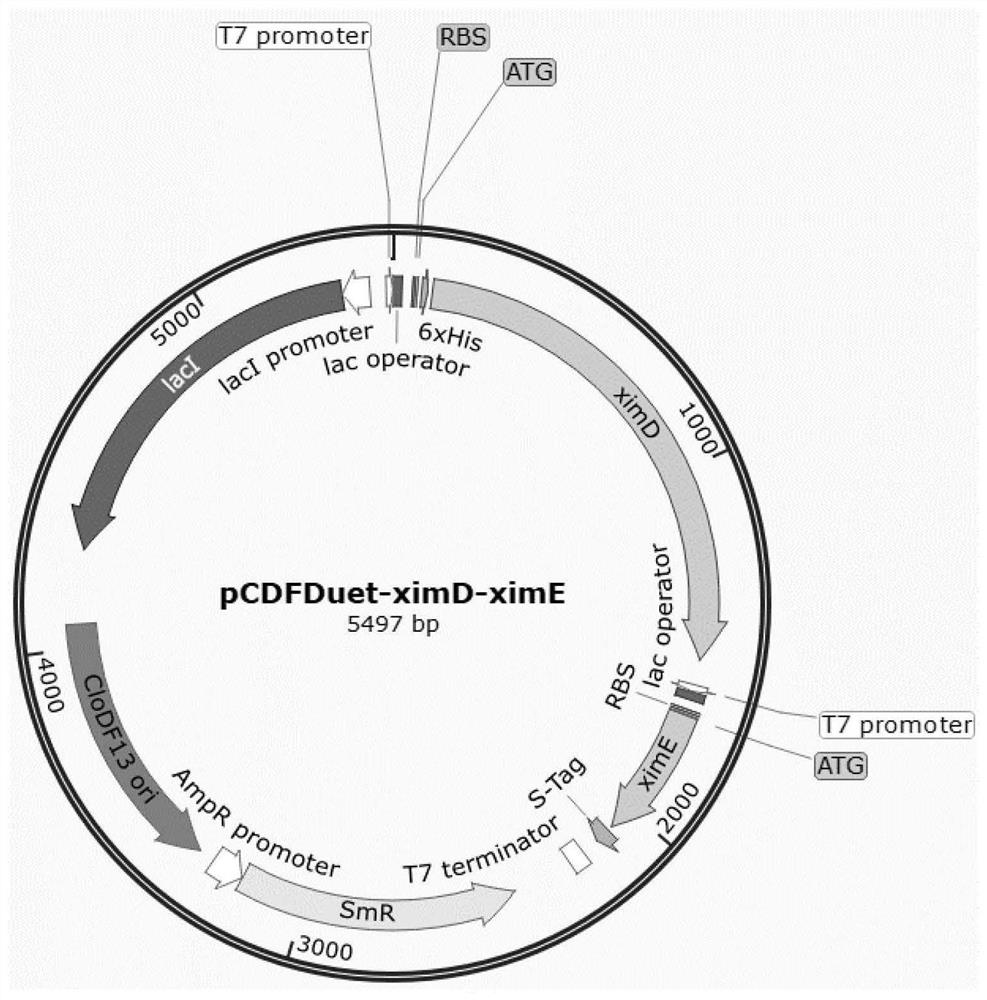
[0035] Plasmid pCDF-Duet-ximD contains 1 gene in the benzopyran biosynthetic gene cluster: the ximD gene (monooxygenase) from Streptomyces xiamenensis 318; Two genes in the synthetic gene cluster: the ximD and ximE genes (cyclase) from Streptomyces xiamenensis 318. All genes were amplified by PCR, wherein the ximD gene was amplified using the Streptomycesxiamenensis 318 genome as a template, and the ximE gene was a gene (ximEsyn) obtained after codon optimization for Escherichia coli, and its sequence is shown in SEQ ID NO.1 .
[0036] The schematic diagram of the heterologous synthesis of Indian quincetin, dihydroeosanthin, Japanese procurerol and lomatin described in the present invention is as follows figure 1 shown.
[0037] Plasmids and primers used in the examples are shown in Table 1:
[0038] Table 1 Primer list
[0039]
[0040]
[0...
Embodiment 2
[0051] Example 2 Construction of Indian quincetin and dihydro-axerinin production strains
[0052] The plasmid pCDF-Duet-ximD was directly transformed into Escherichia coli BL21(DE3) by heat shock calcium transformation method to obtain strain XL01.
Embodiment 3
[0053] The construction of embodiment 3 japonica alcohol and lomatin production strain
[0054] The plasmid pCDF-Duet-ximD-ximE was transformed into Escherichia coli BL21(DE3) by heat shock calcium transformation method to obtain strain XM02.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com