Silver azide initiating explosive film and preparation method thereof
A technology of silver azide and primary explosives, which is applied in the fields of azide/azide/halogen azide, offensive equipment, coating, etc., and can solve problems such as complex synthesis process, high risk, and prominent safety issues. The preparation process is simple and efficient, the operation safety is high, and direct contact is avoided
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Step 1, configure porous silver electrolyte, use water as solvent, composition is 0.01mol / L silver sulfate, 1.5mol / L potassium thiocyanate and 1.0mol / L ammonium chloride; configure azide electrolyte, use water as The solvent is composed of 0.02mol / L sodium azide.
[0027] Step 2, using the electrochemical cathodic deposition method, the conductive substrate is subjected to electrode reaction in the porous silver electrolyte configured in step 1, and the current density is 1.0A / cm 2 , the response time is 30s. After the reaction is finished, it is dried to obtain a porous silver film.
[0028] Step 3, using the electrochemical anode azidation method, using the porous silver film prepared in step 2 as the anode, and performing electrode reaction in the azide electrolyte configured in step 1, with a current density of 1.0mA / cm 2 , the reaction time is 10min. After the reaction is finished, it is dried to obtain a porous silver azide primer film.
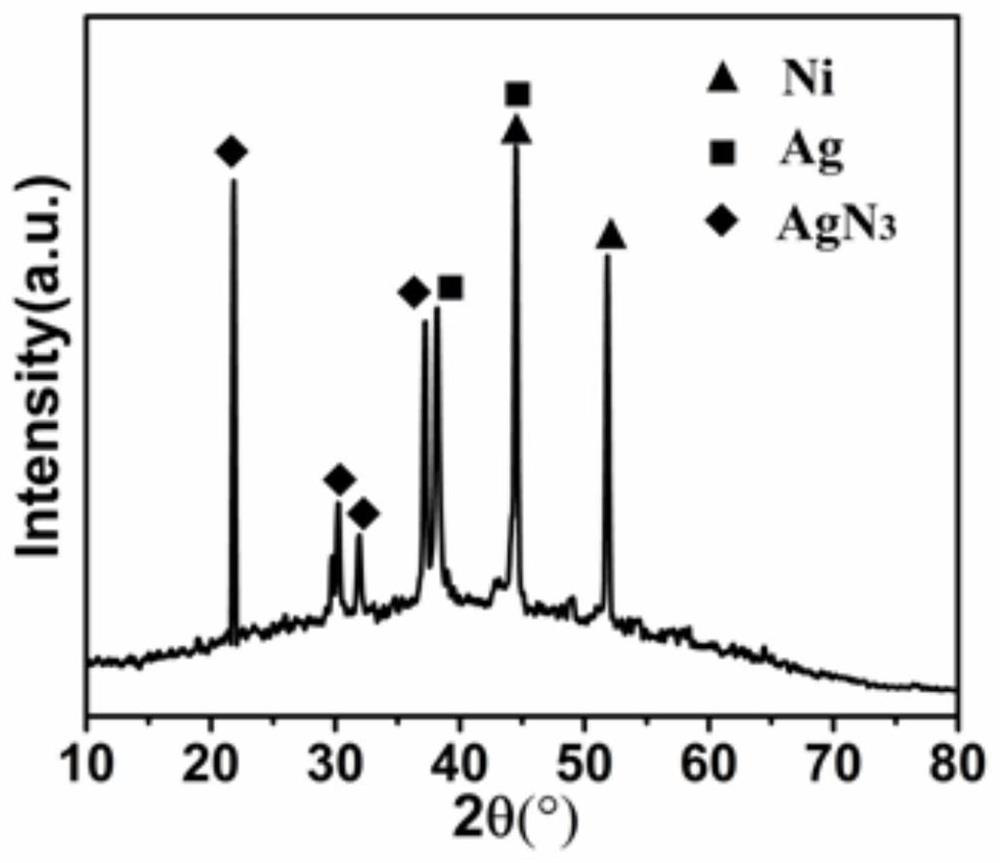
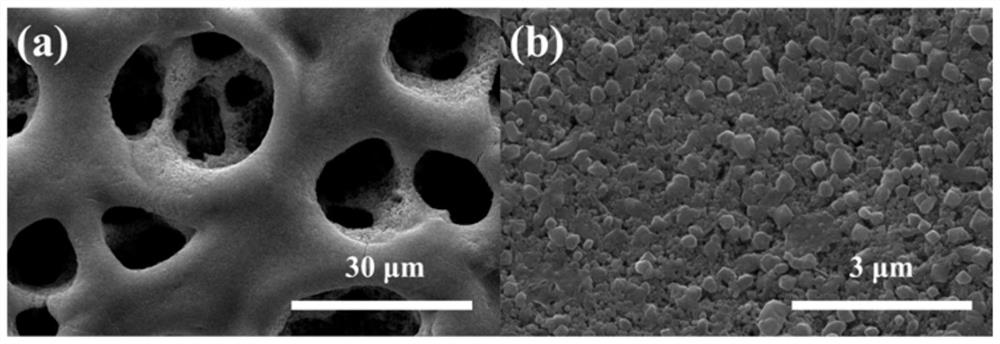
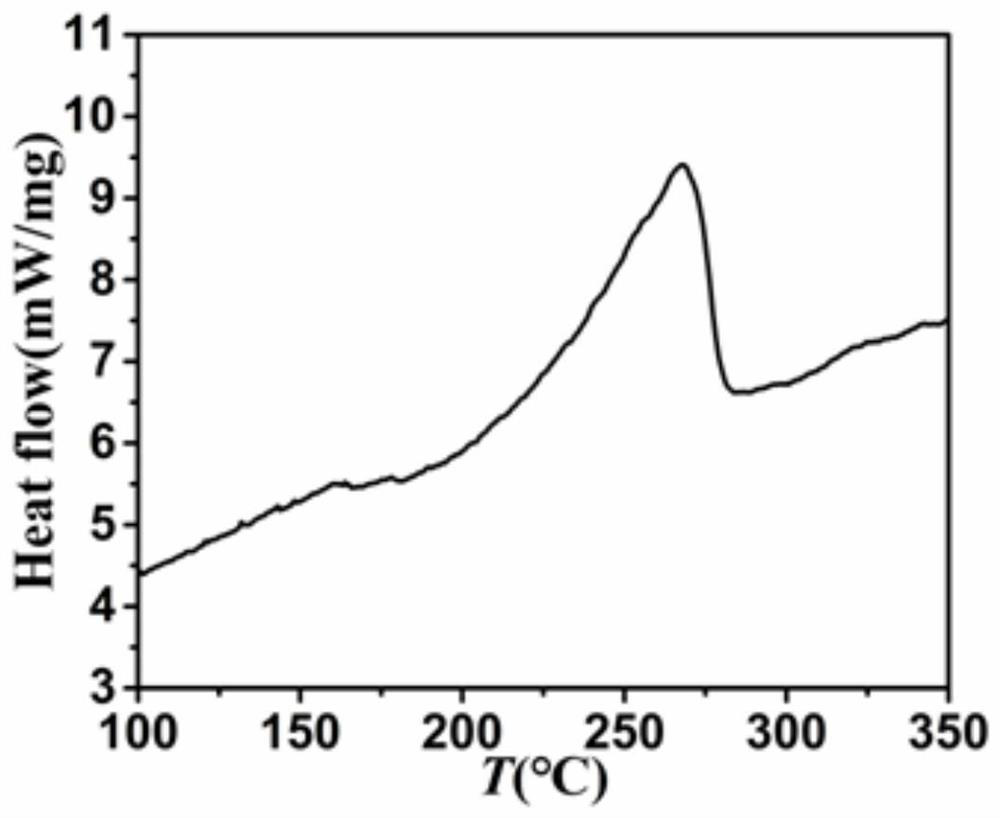
[0029] figure 1 It i...
Embodiment 2
[0033] Step 1, configure a porous silver electrolyte, using water as a solvent, the composition is 0.005mol / L silver sulfate, 0.5mol / L potassium thiocyanate and 0.5mol / L ammonium chloride; configure an azide electrolyte, using water as The solvent is composed of 0.01mol / L potassium azide.
[0034] Step 2, using the electrochemical cathodic deposition method, the conductive substrate is subjected to electrode reaction in the porous silver electrolyte configured in step 1, and the current density is 0.5A / cm 2 , the response time is 60s. After the reaction is finished, it is dried to obtain a porous silver film.
[0035] Step 3, using the electrochemical anode azidation method, using the porous silver film prepared in step 2 as an anode, and performing an electrode reaction in the azide electrolyte configured in step 1, with a current density of 10mA / cm2 , the reaction time is 5min. After the reaction is finished, it is dried to obtain a porous silver azide primer film.
Embodiment 3
[0037] Step 1, configure a porous silver electrolyte, using water as a solvent, the composition is 0.02mol / L silver sulfate, 2mol / L potassium thiocyanate and 2mol / L ammonium chloride; configure an azide electrolyte, using water as a solvent, The composition is 1mol / L sodium azide.
[0038] Step 2, using the electrochemical cathodic deposition method, the conductive substrate is subjected to electrode reaction in the porous silver electrolyte configured in step 1, and the current density is 2A / cm 2 , the response time is 10s. After the reaction is finished, it is dried to obtain a porous silver film.
[0039] Step 3, using the electrochemical anode azidation method, using the porous silver film prepared in step 2 as the anode, performing electrode reaction in the azide electrolyte configured in step 1, and the current density is 0.1mA / cm 2 , The reaction time is 30min. After the reaction is finished, it is dried to obtain a porous silver azide primer film.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Heat release | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com