Preparation method of vonoprazan fumarate intermediate
A compound and solvent technology, applied in the field of preparation of vonoprazan fumarate intermediates, can solve the problems of unsuitable industrial production, increase production costs and the like, and achieve the effects of low price, low production cost and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
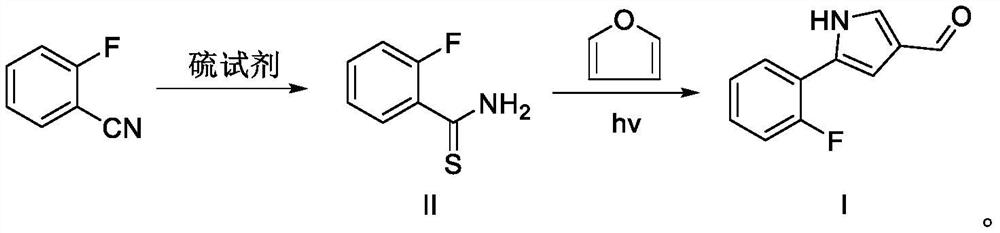
[0052] Add 1,4-dioxane (20mL), o-fluorobenzonitrile (3.63g, 30mmol), triethylamine (3.34g, 33mmol) and 20% aqueous ammonium sulfide (11.2g, 33 mmol). The temperature of the reaction solution was raised to 55 °C and continued to stir for 6 h, then cooled to room temperature. Water was added to the reaction liquid, followed by extraction with ethyl acetate. The combined organic phases were washed with water, dried over anhydrous sodium sulfate, filtered, and concentrated in vacuo to obtain intermediate II (4.61 g, 99%). 1 H NMR (Methanol-d 4, 400MHz) δ8.54-8.50 (m, 1H); 7.48-7.44 (m, 1H); 7.24-7.14 (m, 2H).
Embodiment 2
[0054] Add 1,4-dioxane (20mL), o-fluorobenzonitrile (3.63g, 30mmol), triethylamine (3.34g, 33mmol), sodium sulfide nonahydrate (7.9g, 33mmol) to a 100mL reaction flask in sequence ) and water (10 mL). The temperature of the reaction solution was raised to 55 °C and continued to stir for 6 h, then cooled to room temperature. According to the post-processing method of Example 1, Intermediate II (4.52 g, 97%) was obtained.
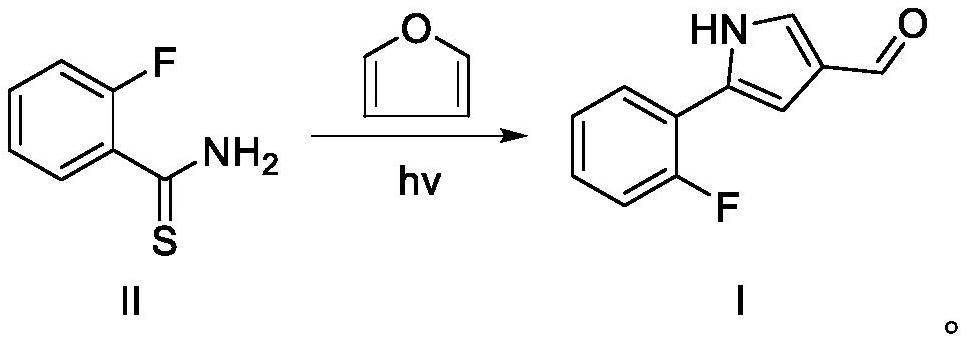
[0055] Preparation of Intermediate I
Embodiment 3
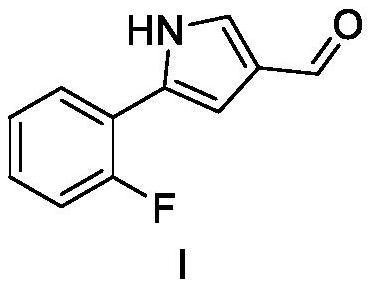
[0057] Intermediate II (15.5 g, 100 mmol), furan (136.1 g, 2 mol) and methanol (500 mL) were sequentially added to a 1000 mL reaction flask. After nitrogen replacement three times, the temperature of the reaction solution was raised to 30-40°C and irradiated with a mercury lamp for 5 hours. Furan and methanol were recovered by distillation, and the remaining residue was recrystallized from ethyl acetate and petroleum ether to obtain intermediate I (15.3 g, 81%). 1 H NMR (CDCl 3,400MHz) δ9.85 (s, 1H); 9.31 (brs, 1H); 7.67-7.62 (m, 1H); 7.56-7.50 (m, 1H); 7.24-7.05 (m, 4H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com