Organic thermally-induced delayed fluorescence material based on pyrazine receptor as well as preparation method and application of organic thermally-induced delayed fluorescence material
A thermally induced delayed fluorescence, organic technology, applied in the direction of luminescent materials, organic chemistry, chemical instruments and methods, etc., can solve the problems of reducing radiation attenuation rate, reducing oscillation intensity, etc., to achieve improved emission efficiency, good thermal stability, and suppression Effects of nonradiative transitions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
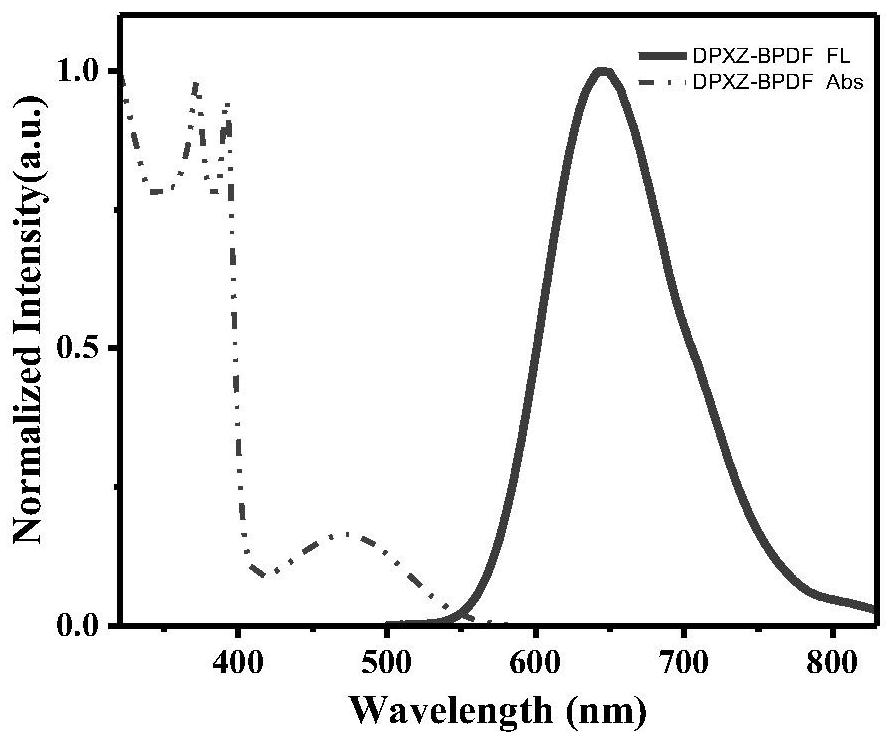
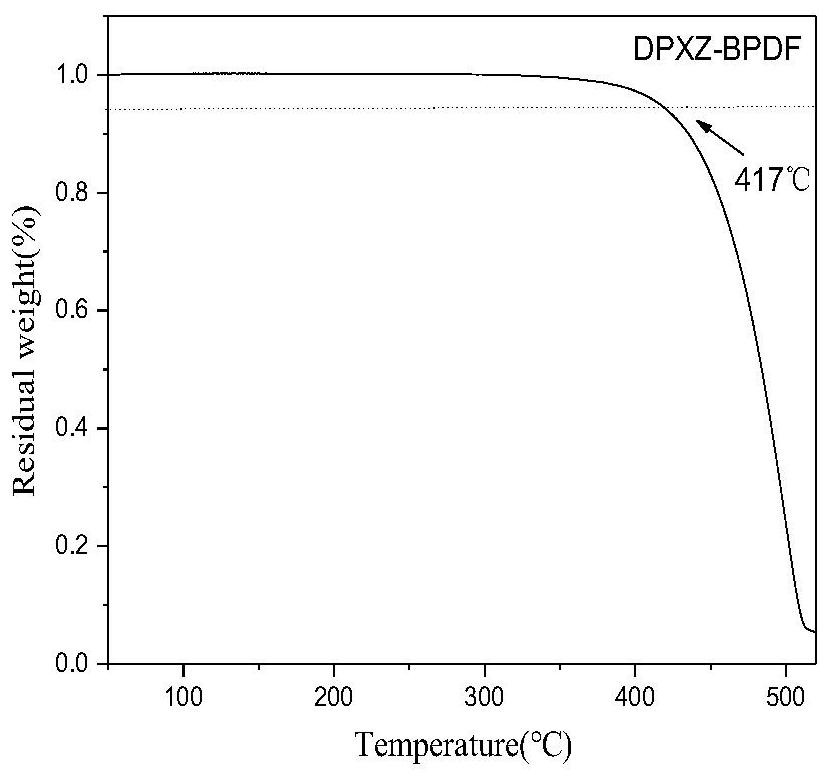
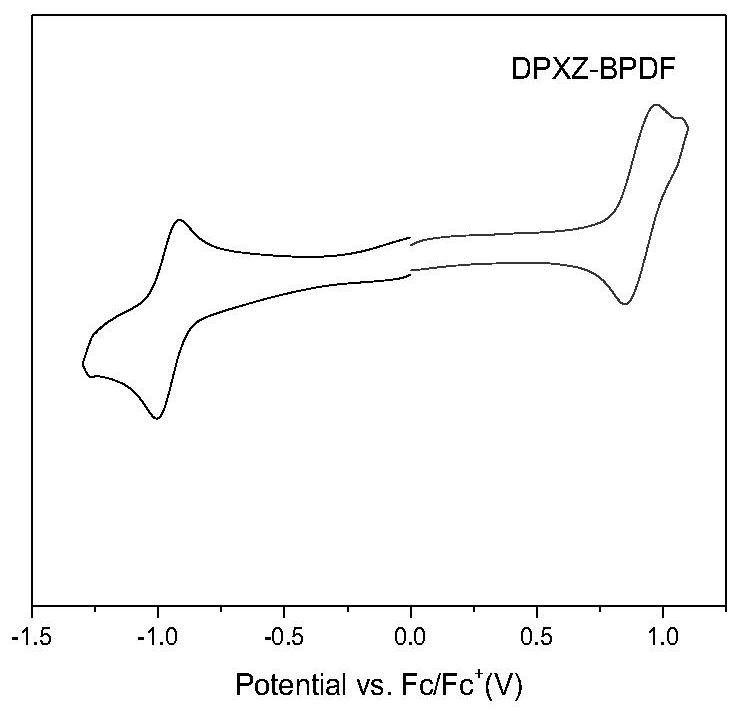
[0069] This example proposes an organic thermally induced delayed fluorescence material based on a pyrazine acceptor, the molecular structure of which is DPXZ-BPDF as follows:
[0070]
[0071] The synthetic route is as follows:
[0072]
[0073] Specifically include the following steps:
[0074] Step 1: Add 3,6-dibromophenanthrenequinone (366mg, 1mmol), phenoxazine (420mg, 2.3mmol), and palladium acetate (8.42mg, 0.04mmol) to a 100mL two-necked round-bottom flask with a magnet in sequence , sodium tert-butoxide (217mg, 2.25mmol) and tri-tert-butylphosphine tetrafluoroborate (29mg, 0.1mmol), the nitrogen was replaced three times, and under the protection of nitrogen, 30mL of toluene was extracted with a 50mL syringe and added to the round bottom flask , rotate the reaction at an oil bath temperature of 110°C; use thin-layer chromatography (TCL) to spot plate detection, wait until 3,6-dibromophenanthrenequinone has completely reacted (reaction 20h), and use a Buchner fun...
Embodiment 2
[0086] This example proposes an organic thermally induced delayed fluorescence material based on a pyrazine acceptor, and the molecular structure is TPXZ-BPF as shown below:
[0087]
[0088] The synthetic route is as follows:
[0089]
[0090] Specifically include the following steps:
[0091] Step 1: Add phenoxazine (183mg, 1.1mmol) and sodium hydride (36mg, 1.5mmol) successively to the first 100mL two-necked round-bottomed flask with a magnet, replace the nitrogen three times, under the protection of nitrogen, Use a 50mL syringe to extract 20mL of ultra-dry N,N-dimethylformamide into a round bottom flask under the condition of an ice bath. After the reaction solution in the round bottom flask turns dark green, remove the ice bath and raise the temperature to 70 °C to obtain a mixed solution D;
[0092] Step 2: Add the pyrazine acceptor-based organic thermally induced delayed fluorescent material (DPXZ-BPDF) (678mg, 1mmol) obtained in Example 1 to the second 100mL ro...
Embodiment 3
[0102] This example proposes an organic thermally induced delayed fluorescence material based on a pyrazine acceptor, the molecular structure of which is DPXZ-BPDPA as shown below:
[0103]
[0104] The synthetic route is as follows:
[0105]
[0106] Specifically include the following steps:
[0107] Step 1: Add intermediate A (570mg, 1mmol) and 4-bromo-o-phenylenediamine (190mg, 1.1mmol) successively to a 100mL two-necked round-bottomed flask with a magnet, and replace the nitrogen three times. , use a 50mL syringe to extract 30mL of acetic acid and add it to a round bottom flask, and rotate the reaction at an oil bath temperature of 110°C; use a TCL spot plate to detect, wait until the intermediate A is completely reacted (reaction 8h), use a separatory funnel to separate the reaction system Extract the medium acetic acid (with dichloromethane and water), then wash the funnel with 200mL of dichloromethane, add 200-300 mesh thick silica gel to the filtrate and distill...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thermal decomposition temperature | aaaaa | aaaaa |
| thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com