Method for preparing 2-iodo aryl ether under action of alkali metal hydride
A technology of alkali metal hydrides and aryl ethers, which is applied in the preparation of organic compounds, metal/metal oxide/metal hydroxide catalysts, ester reaction to prepare ethers, etc., to achieve cheap reagents, high compatibility, and simple reactions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027]
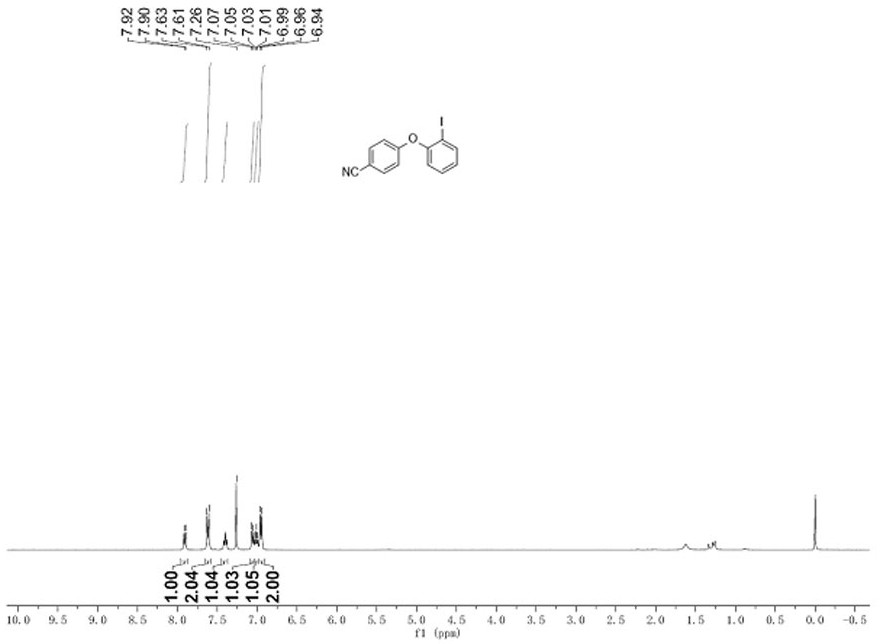
[0028] Sodium hydride (60% in oil, 36 mg, 0.9 mmol, 3 eq.) was suspended in THF (1 mL), and phenol 2a (36 mg, 0.3 mmol, 1 eq.) was added in DMA (0.3 mL ) solution, stirred at room temperature for 10 minutes after the addition was completed, then added a solution of 1a (148 mg, 0.45 mmol, 1.5 eq.) in THF (0.2 mL), reacted at room temperature for 2 hours, quenched with water, and then dissolved in ethyl acetate The ester was extracted three times, the organic layers were combined, dried with sodium sulfate, evaporated to dryness, and purified by column chromatography to obtain the product iodoaryl ether 3aa with a yield of 99%. figure 1 , LR-MS (ESI): m / z 322.1 [M+H] + .
[0029]
[0030] The above-mentioned sodium hydride was replaced with potassium hydride in an equimolar amount, and the rest remained unchanged to obtain the product iodoaryl ether 3aa with a yield of 39%.
[0031] Replace the above-mentioned sodium hydride with lithium hydride in an equimolar...
Embodiment 2
[0037]
[0038] Sodium hydride (60% in oil, 36 mg, 0.9 mmol, 3 eq.) was suspended in THF (1 mL), and phenol 2a (28 mg, 0.3 mmol, 1 eq.) was added in DMA (0.3 mL ) solution, stirred at room temperature for 10 minutes after the addition was complete, then added a solution of 1a (148 mg, 0.45 mmol, 1.5 eq.) in THF (0.2 mL), reacted at room temperature for 1 hour, quenched with water, washed with ethyl acetate The ester was extracted three times, the organic layers were combined, dried over sodium sulfate, evaporated to dryness, and purified by column chromatography to obtain the product iodoaryl ether 3ba with a yield of 35%.
Embodiment 3
[0040]
[0041] Sodium hydride (60% in oil, 36 mg, 0.9 mmol, 3 eq.) was suspended in THF (1 mL), and phenol 2b (42 mg, 0.3 mmol, 1 eq.) was added in DMA (0.3 mL ) solution, stirred at room temperature for 10 minutes after the addition was complete, then added a solution of 1a (148 mg, 0.45 mmol, 1.5 eq.) in THF (0.2 mL), reacted at room temperature for 0.5 hours, quenched with water, washed with ethyl acetate The ester was extracted three times, the organic layers were combined, dried over sodium sulfate, evaporated to dryness, and purified by column chromatography to obtain the product iodoaryl ether 3ab with a yield of 97%. 1 H NMR (400 MHz, CDCl 3 ) δ: 8.22 (d, J =7.8 Hz, 2H), 7.92 (d, J = 7.5 Hz, 1H), 7.43 (t, J = 7.5 Hz, 1H), 7.09 (d, J =7.5 Hz, 1H), 7.03 (t, J = 7.2 Hz, 1H), 6.96 (d, J = 7.8 Hz, 2H). LR-MS (ESI): m / z 342.1 [M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com