Preparation process of ferric carboxymaltose injection
A carboxymaltose iron and preparation process technology, which is applied in the direction of medical preparations containing active ingredients, blood diseases, extracellular fluid diseases, etc., can solve the unseen continuous production process of carboxymaltose iron, the influence of carboxymaltose iron molecular weight, and large consumption Solvent and energy problems, to achieve the effect of saving solvent and energy, easy to realize, and high practicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Add 200 mL of water to 100 g of maltodextrin and stir to dissolve; add 30% sodium hydroxide solution to adjust the pH of the maltodextrin aqueous solution to 9.0-11.0, control the temperature at 25-40°C, and add 80 g of 10% sodium hypochlorite solution under stirring to obtain carboxylated malt Dextrin solution.
[0025] Preparation of ferric chloride solution: 220 g of ferric chloride hexahydrate was mixed with water to form a 75% (w / w) solution.
[0026] Preparation of sodium carbonate solution: add 110 g of sodium carbonate to prepare a 25% (w / w) solution.
[0027] The carboxy maltodextrin solution and the ferric chloride solution are first mixed, stirred, the temperature is controlled at 50-70°C, the flow rate is set, and the sodium carbonate solution is added dropwise at a constant speed with a peristaltic pump, and the sodium carbonate solution is controlled to be added within 1.0 hour. After completion, carry out alkali curing, acid curing and high temperature c...
Embodiment 2
[0039] According to the technical process of Example 1, each material was enlarged by 2 times to obtain a stable iron carboxy maltose solution.
[0040] Add 320 g of absolute ethanol to carboxymaltose iron solution to precipitate carboxymaltose iron, filter, add 1200 mL of water for injection to the filter cake, stir to dissolve, adjust the pH value to 6.5 with 30% sodium hydroxide solution, and measure iron by complexometric titration content, calculate the amount of water replenishment, add water to 1866mL, and retest the iron content to be 49.12mg / mL; then filter through a 0.22μm filter membrane, fill the filtrate into 2mL ampoules, and sterilize.
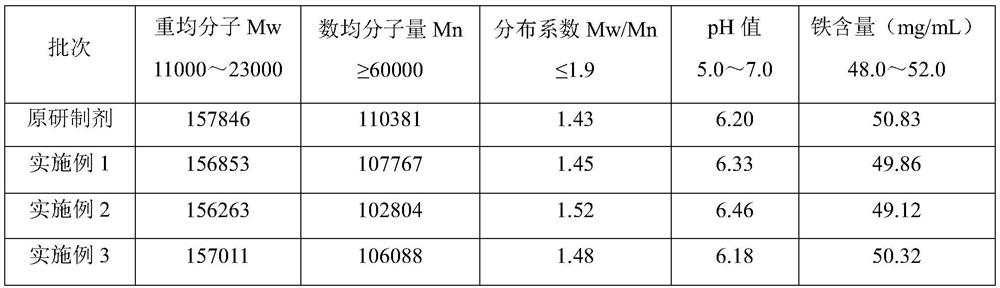
[0041] According to the method of Example 1, the molecular weight and iron content of carboxymaltose iron in the present embodiment carboxymaltose iron injection were determined. See Table 1, the key indicators of this embodiment are consistent with those of the original preparation.
Embodiment 3
[0043] According to the technical process of Example 1, each material was enlarged 4 times to obtain a stable iron carboxy maltose solution.
[0044] Add 640g of absolute ethanol to carboxymaltose iron solution to precipitate carboxymaltose iron, filter, add 2400mL of injection to the filter cake, stir to dissolve, adjust the pH value to 6.3 with 30% sodium hydroxide solution, and use complexometric titration to measure iron content, calculate the amount of water replenishment, add water to 3629mL, and retest the iron content to 50.32mg / mL; then filter through a 0.22μm filter membrane, fill the filtrate into 2mL ampoules, and sterilize.
[0045] According to the method of Example 1, the molecular weight and iron content of carboxymaltose iron in the present embodiment carboxymaltose iron injection were determined. See Table 1, the key indicators of this embodiment are consistent with those of the original preparation.
[0046] Table 1: Key indicators of embodiment 1-embodimen...
PUM
| Property | Measurement | Unit |
|---|---|---|
| distribution coefficient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com