Recombinant escherichia coli for producing L-glutamine and construction method and application thereof
A technology for recombining Escherichia coli and glutamine, which is applied in the field of genetic engineering, can solve the problems of further improvement of the titer of fermentation method, increase of glutamine refining cost, complicated separation and purification steps, etc., and achieve single reaction system components and high yield The effect of high, short synthetic route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0179] Embodiment 1, construct the recombinant plasmid of overexpressing glutamine synthetase
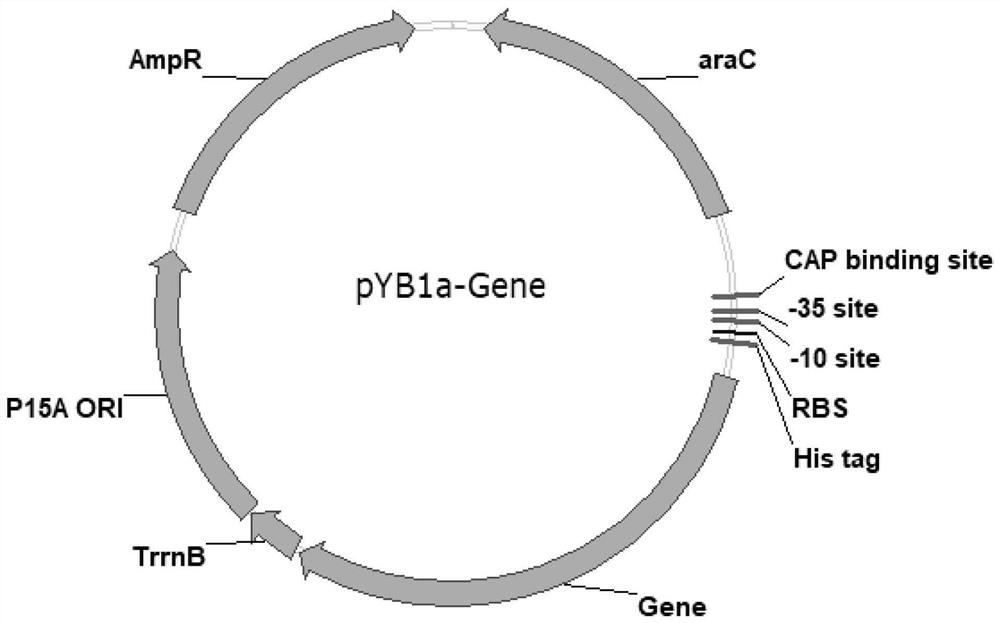
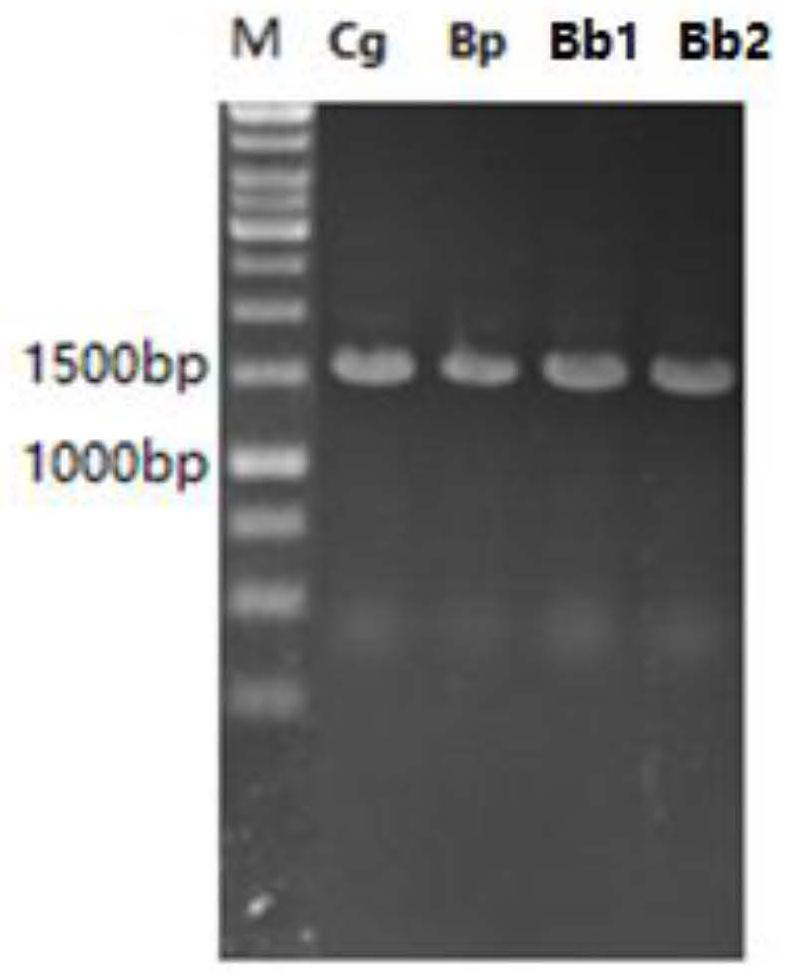
[0180] 1. Gene CgglnA (nucleotide sequence shown in SEQ ID No.1), BpglnA (nucleotide sequence shown in SEQ ID No.2), Bb1glnA (nucleotide sequence shown in SEQ ID No.3), Bb2glnA (nucleotide sequence shown in SEQ ID No.4) was synthesized by Nanjing GenScript Biotechnology Co., Ltd. The gene fragments were respectively inserted between Xho I and EcoR I of the pYB1a vector after double digestion with Xho I and EcoR IHF, and the ligation product was transformed into Trans1-T1 competent cells (Beijing Quanshijin Biology, catalog number CD501), Spread LB solid plates containing the corresponding ampicillin resistance. Overnight at 37°C, single clones were picked to extract plasmids, and primer pairs P1 (5'-cggcgtcacactttgctatg-3') and P2 (5'-cgtttcacttctgagttcggc-3') were used for PCR verification, and the correct clones were sent for sequencing. The recombinant plasmids with correct seq...
Embodiment 2
[0185] Example 2, construction of L-glutamine-producing Escherichia coli mutants AQ02, AQ04, AQ06
[0186] Use CRISPR-Cas9 gene knockout technology to edit wild-type E. coli K12 to construct E. coli mutants: the Genbank number of knockout E. coli K12 strain BW25113 is NC_000913.3 (update date is 10-Oct-2019) The coding gene (glsA) of glutaminase A shown in the 511641-512573 positions obtained the Escherichia coli mutant AQ03, and the genotype was BW△GlsA; the Genbank number of the knockout Escherichia coli K12 bacterial strain BW25113 was No. 2687070 of NC_000913.3 The coding gene (glnB) of the PII-1 protein shown in -2687408, the GlnE shown in the 3196801-3199641 of Genbank No. NC_000913.3 (having both glutamine synthetase adenyltransferase activity (ATase) and The coding gene (glnE) of the bifunctional enzyme of glutamine synthetase deadenylation activity (ATd) obtains Escherichia coli mutant AQ02, and the genotype is BW△GlnEB; Knockout the SEQ ID No of Escherichia coli K12 ...
Embodiment 3
[0221] Embodiment 3, construct the recombinant escherichia coli producing L-glutamine
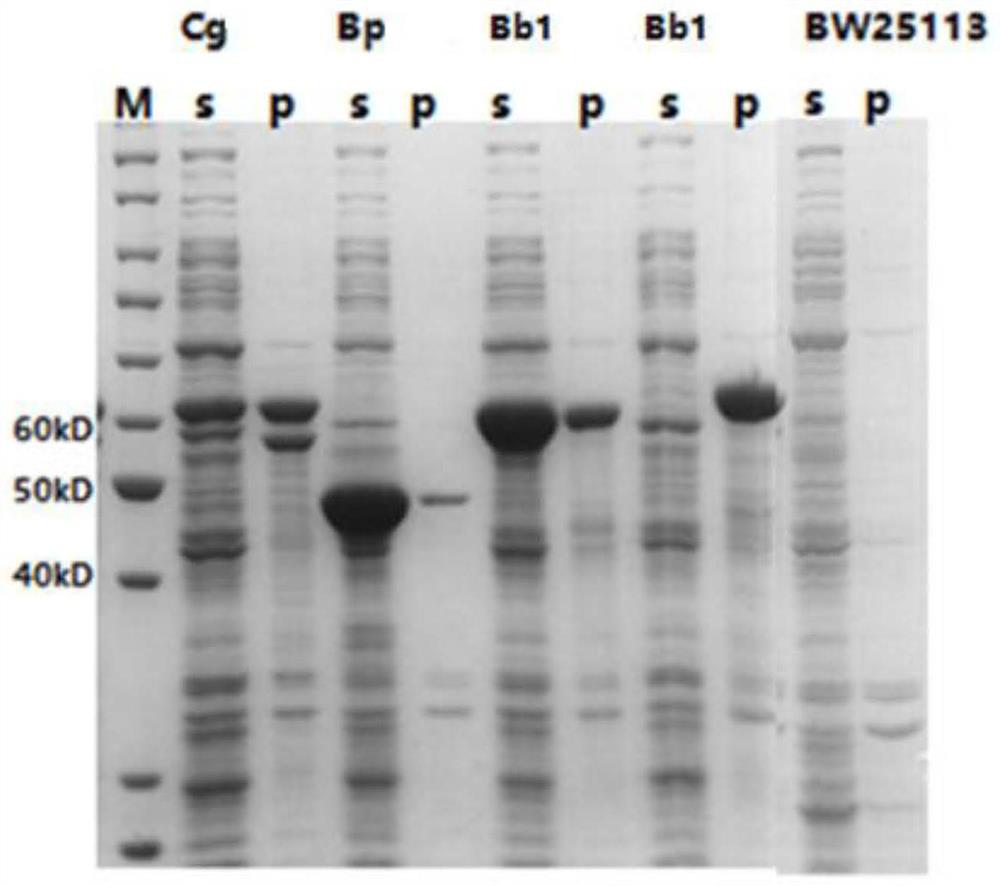
[0222] According to Table 2, the recombinant plasmids prepared in Example 1 were respectively transformed into Escherichia coli mutants AQ02, AQ04, AQ06 and Escherichia coli BW25113 constructed in Example 2 by the calcium chloride method. ml) resistant LB plate to screen positive clones (clones that can grow on the plate) to obtain recombinant Escherichia coli as shown in Table 2.
[0223] Table 2 shows the information of each recombinant Escherichia coli used in the above L-glutamine yield detection.
[0224] Table 2. Information of each recombinant Escherichia coli
[0225] recombinant plasmid host bacteria Recombinant Escherichia coli pYB1a-CgGlnA Escherichia coli K12 strain BW25113 pYB1a-CgGlnA / BW pYB1a-CgGlnA Escherichia coli mutant AQ02 pYB1a-CgGlnA / AQ02 pYB1a-CgGlnA Escherichia coli mutant AQ04 pYB1a-CgGlnA / AQ04 pYB1a-CgGlnA Escherichi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com