Preparation process of 5-fluoro-2-hydroxyacetophenone
A technology for hydroxyacetophenone and preparation process is applied in the field of preparation technology of 5-fluoro-2-hydroxyacetophenone, and can solve the complicated separation and purification of intermediate product ester, the influence of acylation and rearrangement yield, and the expensive synthesis raw materials and other problems, to achieve the effect of convenient disassembly and assembly, prolonged residence time, and simple structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
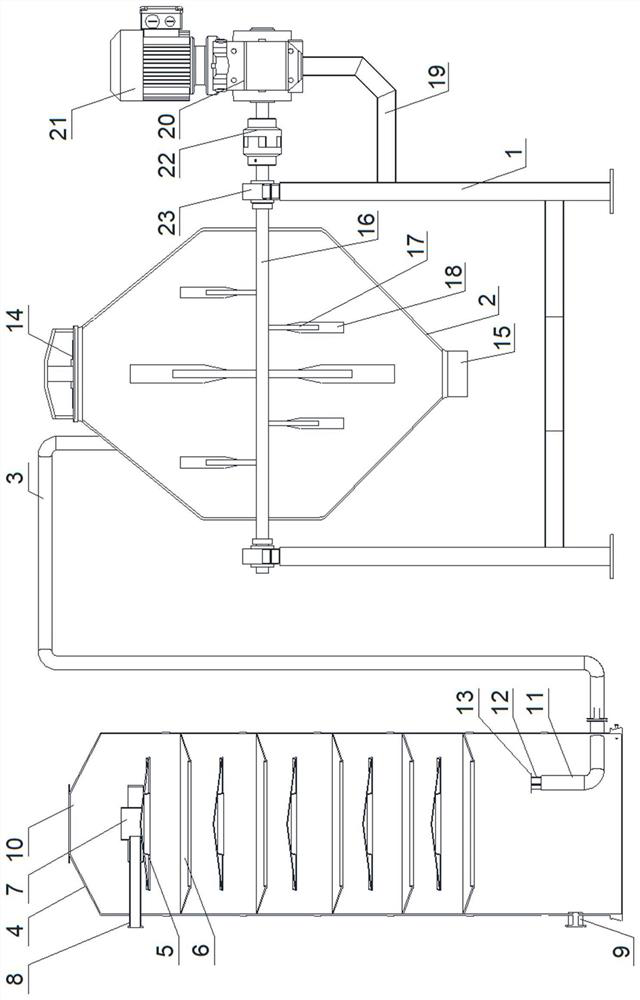
[0033] A preparation process of 5-fluoro-2-hydroxyacetophenone, comprising the steps of:
[0034] Step 1, aminophenol, acetic anhydride and benzene prepare 4-acetamidophenol acetate;
[0035] Step 2, putting anhydrous aluminum chloride, sodium chloride and the 4-acetamidophenol acetate gained in step 1 into the stirring device to prepare 2-acetyl-4-acetamidophenol;
[0036] Step 3, hydrochloric acid, reflux of 2-acetyl-4-acetamidophenol obtained in step 2, cooling, adjusting the pH value to 6-7, precipitating solids, and drying to prepare 2-acetyl-4-aminobenzene;
[0037] Step 4. Add the 2-acetyl-4-aminophenol obtained in Step 3 to excess hydrogen fluoride, drop the temperature to -5°C, gradually add solid sodium nitrite, and keep the reaction temperature at 0-5°C. After the addition, keep React for 5-8 hours, then gradually raise the temperature to 40°C for thermal decomposition. After the decomposition is complete, adjust the pH value to 6-7, steam distillation to obtain a ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com