Bacterial strain as well as composition and application
A composition and strain technology, applied in the direction of drug combination, bacterial antigen components, drug delivery, etc., can solve problems such as adverse reactions of patients and drugs that cannot achieve the expected effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
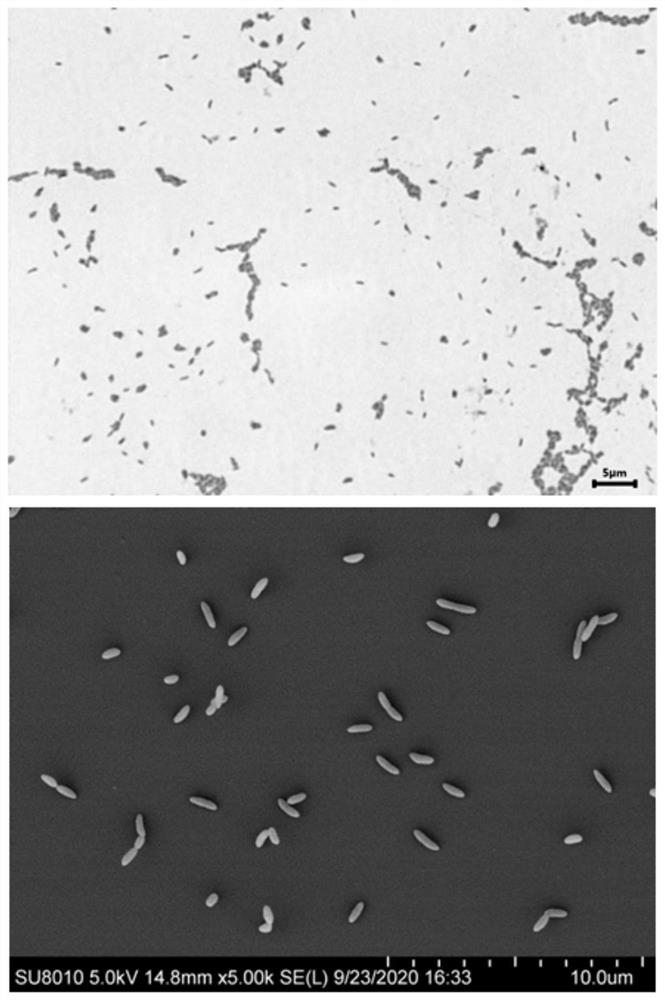
[0107] This example provides the isolation and identification of Christensenella sp. MNO-863.
[0108] (1) Isolation of MNO-863
[0109] Christensenella sp.MNO-863 of the present invention is isolated from a stool sample of a healthy male volunteer of Han nationality in Guangzhou City, Guangdong Province. The volunteer did not use antibiotics in the previous three months when the sample was collected.
[0110] Dispense normal saline into sterile 10ml centrifuge tubes in a biological safety cabinet; transfer the anaerobic blood plate (Jiangmen Kailin anaerobic blood agar medium, Guangdong Mechanical Note 20172400940) and sterile normal saline into an anaerobic tube 24 hours in advance. In the oxygen workbench, pour 5-7 sterile glass beads into the solidified anaerobic blood plate.
[0111] Take an appropriate amount of fresh stool samples from volunteers and store them in a sample containing sterile preservation solution (3% PEG solution, that is, weigh 30g of polyethylene gly...
Embodiment 2
[0135] In this example, an in vivo test of MNO-863 in a high-fat diet-induced obese mouse model was carried out to verify its use in the treatment or prevention of liver function damage and related diseases.
[0136] Experimental Materials:
[0137] (1) Experimental animals: 32 C57BL / 6J male mice (purchased from Jiangsu Jicui Yaokang Biotechnology Co., Ltd.) were purchased, which were normally fed mice and were 5 weeks old. The mice were kept in the same environment during the growth process, and 8 of them were given SPF-grade rat maintenance feed (Guangzhou Hancheng Experimental Equipment Co., Ltd.), and the remaining 24 were fed with D12492 high-fat feed (Pike Biological) for about 8 to 10 weeks. Weighing, diet-induced obesity model modeling standard is that the body weight reaches 38.00±2.00g.
[0138] (2) Tested bacterial strain: MNO-863 of anaerobic culture, culture medium is 104 liquid culture medium in embodiment 1, cultivates 48h under 37 ℃ of anaerobic conditions, to...
Embodiment 3
[0165] In this example, an in vivo test of MNO-863 in an obese mouse model induced by a high-fat diet was carried out to verify its use in treating or preventing diabetes. Experimental Materials:
[0166] (1) Experimental animals: 40 C57BL / 6J male mice (purchased from Jiangsu Jicui Yaokang Biotechnology Co., Ltd.) were purchased, which were normally reared mice and were 5 weeks old. The mice were kept in the same environment during the growth process. Eight of them were given SPF-grade rat maintenance feed (Guangzhou Hancheng Experimental Equipment Co., Ltd.), and 32 were given D12492 high-fat feed (Pike Bio) for about 8 to 10 weeks, and then weighed. , Diet-induced obesity model modeling standard is to reach a body weight of 38.00±2.00g.
[0167] (2) Tested strain: MNO-863 cultured in anaerobic mode, the medium is 104 liquid medium, cultivated under anaerobic conditions at 37°C for 48 hours, until the bacterial concentration is about 10 11 The order of CFU / mL can be used as...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com