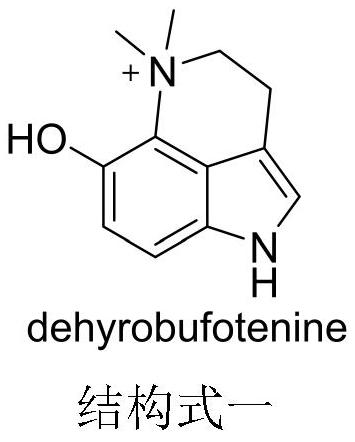
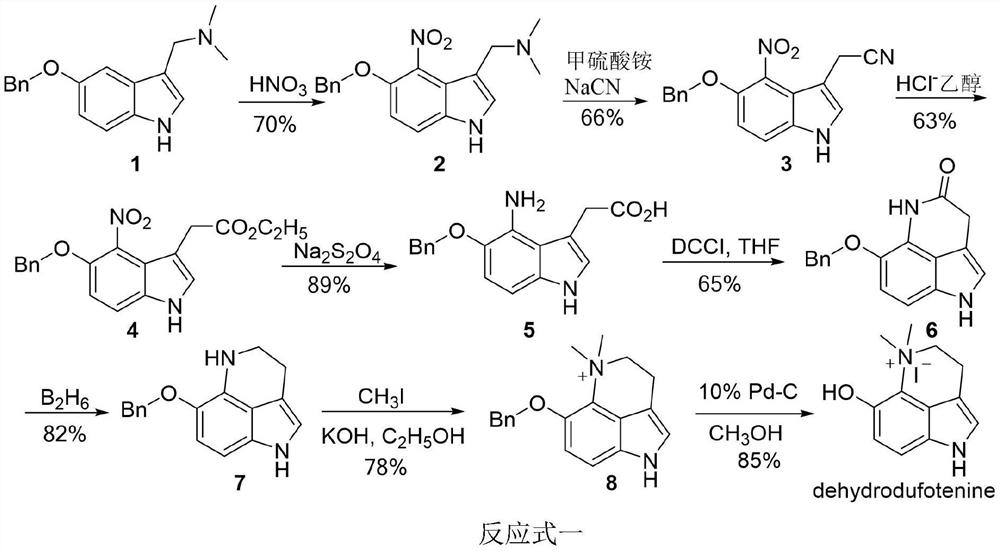
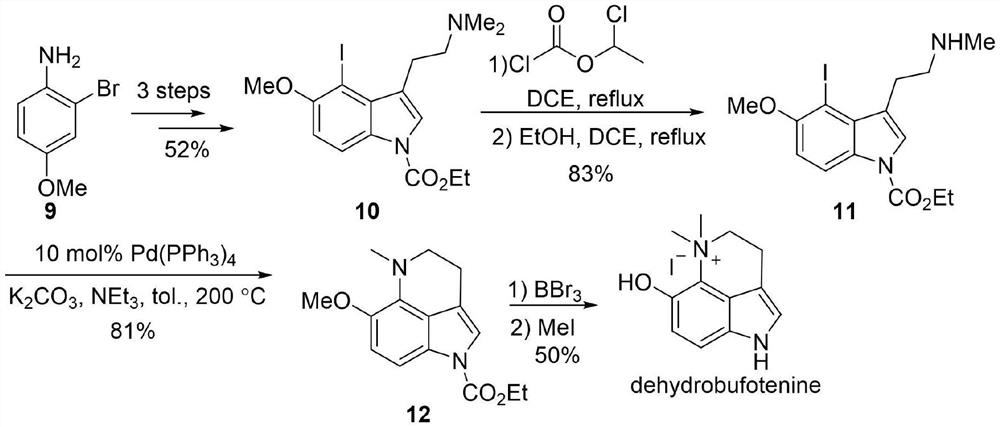
Preparation method and application of dehydrobufotenine
A technology of dehydrobufatryptamine and tetrahydropyrrole, applied in the field of preparation of dehydrobufatryptamine, which can solve the problems of environmental pollution, high production cost, low yield, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0052] The reaction formula of preparation method of the present invention is:
[0053]
[0054]The compound 29 is 2-(5-methoxy-1H-indol-3-yl)-2-oxoacetic acid methyl ester; the compound 30 is 2-(5-methoxy-4-nitro -1H-indol-3-yl)-2-oxoacetic acid methyl ester; The compound 31 is 2-(5-methoxy-4-nitro-indoline-3-yl) methyl acetate, The compound 32 is 6-methoxy-1,2,2a,5-tetrahydropyrrole[4,3,2-de]quinolin-4(3H)-one, and the compound 33 is 6-methoxy Base-1,2,2a,3,4,5-hexahydropyrrole[4,3,2-de]quinoline, the compound 34 is 6-methoxy-2a,3,4,5-tetrahydro Pyrrole [4,3,2-de] quinoline-1 (2H) tert-butyl carboxylate), the compound 35 is 6-methoxy-4,5-dihydropyrrole [4,3,2-de] Quinoline-1 (3H) tert-butyl formate), said compound dehydrobufatryptamine is iodide 6-methoxy-5,5-dimethyl-1,3,4,5-tetrahydropyrrole [ 4,3,2-de]quinoline.
[0055] The first step, using 5-methoxyindole as a raw material to obtain compound 2-(5-methoxyl-1H-indol-3-yl)-2-oxoacetic acid methyl ester through acyl...
Embodiment 1
[0057] The concrete steps of its preparation method are as follows:
[0058] In the first step, add 5-methoxyindole (1.00g, 6.80mmol) and diethyl ether (14mL) into a 100mL round-bottomed flask, add oxalyl chloride (0.68mL) dropwise at 0°C, react for 1.5h and then filter with suction , the solid obtained by suction filtration was added to a 100mL round-bottomed flask, and MeOH (60mL) was added at 0°C, and after reaction for 1h, suction filtration was obtained to obtain compound 29, a yellow solid, with a yield of 98%; The relevant parameters are: 1 H NMR (400MHz, DMSO-d 6 )δ12.32(s,1H),8.37(d,J=3.4Hz,1H),7.66(s,1H),7.45(d,J=8.8Hz,1H),6.92(dd,J=8.8,2.6 Hz,1H),3.89(s,3H),3.80(s,3H); 13 C NMR (100MHz, DMSO-d 6 )δ179.05(s),164.55(s),156.65(s),138.72(s),131.90(s),126.91(s),114.00(s),113.92(s),112.77(s),103.53( s), 55.79(s), 52.95(s), confirm that compound 29 is methyl 2-(5-methoxy-1H-indol-3-yl)-2-oxoacetate;
[0059] In the second step, compound 29 (1.50g, 6.44mmol) was added...
Embodiment 2
[0067] The assay of the anti-tobacco mosaic virus activity of above-mentioned dehydrobufatryptamine, assay procedure is as follows:
[0068] The first step, tobacco mosaic virus purification and concentration determination:
[0069] Tobacco mosaic virus purification and concentration determination were carried out in accordance with the Tobacco Mosaic Virus SOP specification compiled by the Bioassay Laboratory of the Institute of Elements, Nankai University. After the virus crude extract was centrifuged twice with polyethylene glycol, the measured concentration was 20 μg / mL, and refrigerated at 4 °C spare;
[0070] In the second step, the preparation of the above-mentioned dehydrobufatryptamine pharmaceutical solution:
[0071] Weigh 40 mg of dehydrobufatryptamine as the original drug, and then add 0.4 mL of DMF to each of the original drugs for dissolution to obtain 1 × 10 5 μg / mL mother liquor, and then diluted with Tween 80 aqueous solution with a mass percentage concentr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com