Preparation method and application of Pt-based alloy/MOFs catalyst with high hydrogenation selectivity
A catalyst and base alloy technology, applied in the field of molecular biology, can solve the problems of restricting large-scale synthesis, many synthesis steps, and difficulty in ensuring recyclability, and achieve high-efficiency selective hydrogenation performance, unique product structure, and good application prospects. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Example 1 Preparation of PtFe / UiO-66-NH 2 catalyst
[0061] (1)UiO-66-NH 2 Preparation: Add 52mg of zirconium tetrachloride and 39.5mg of 2-aminoterephthalic acid into 50mL of DMF, then add 6mL of glacial acetic acid, stir at room temperature for 30min, transfer the mixed solution to the inner tank of the reactor, and then add polytetrafluoroethylene Put the liner of the ethylene reactor into a high-pressure reactor, and react at 120°C for 12 hours under high temperature and high pressure. ℃ under vacuum to obtain UiO-66-NH 2 .
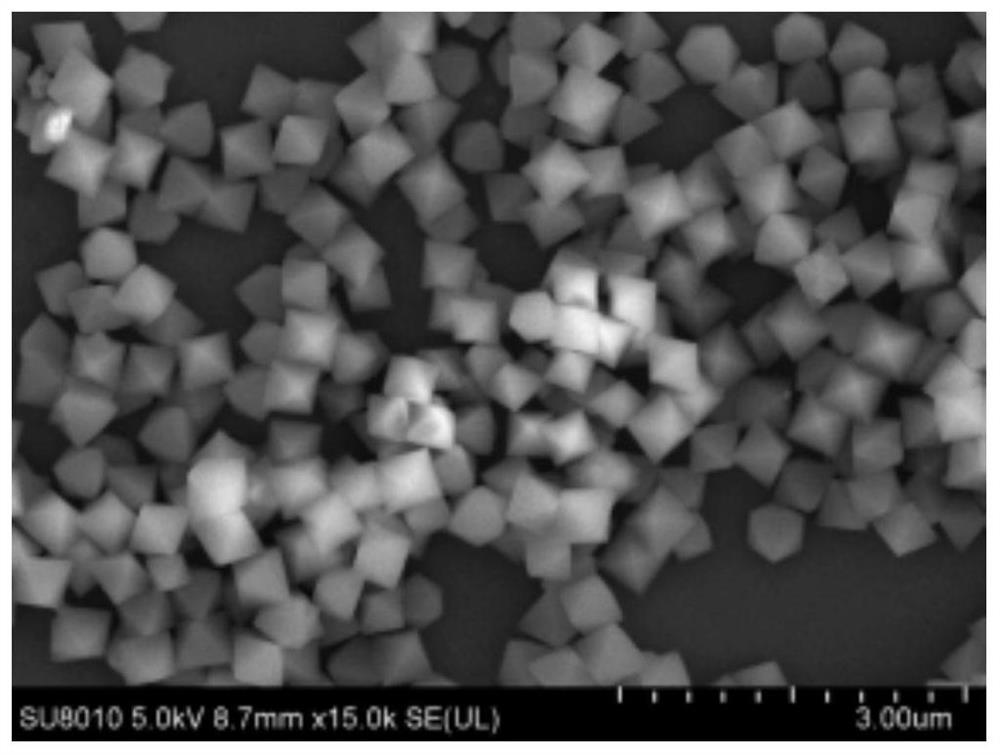
[0062] For the prepared UiO-66-NH 2 Observation was performed with a scanning electron microscope (Hitachi SU8010). Such as figure 1 As shown, the MOF presents an octahedral structure with uniform size.
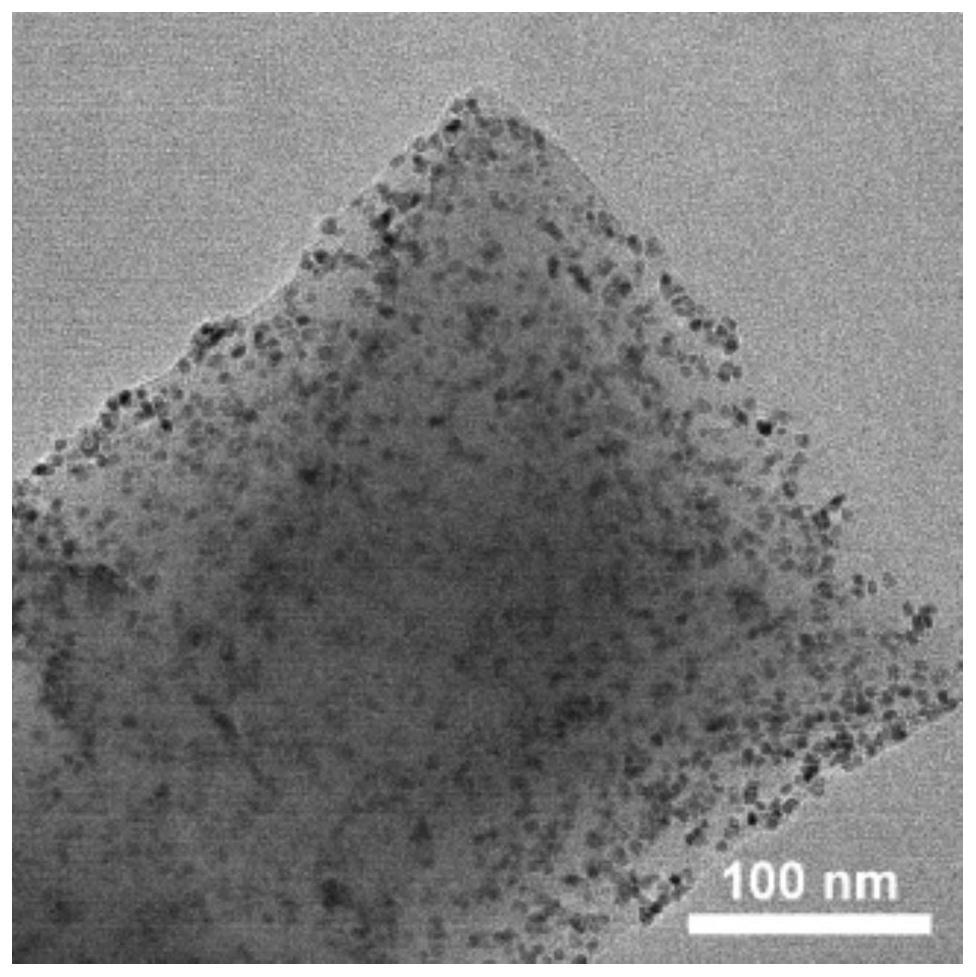
[0063] (2)PtFe / UiO-66-NH 2 Preparation: 50mg UiO-66-NH 2 Add it into 5mL DMF, sonicate until it is uniformly dispersed (360W power, sonicate for 5 minutes), stir at room temperature for 30min to obtain solution A; then add 0.01mmol pl...
Embodiment 2
[0068] Embodiment 2 prepares PtM 2 / UiO-66-NH 2 (M=Ni or Co) catalyst
[0069] (1)UiO-66-NH 2 The preparation is with embodiment 1.
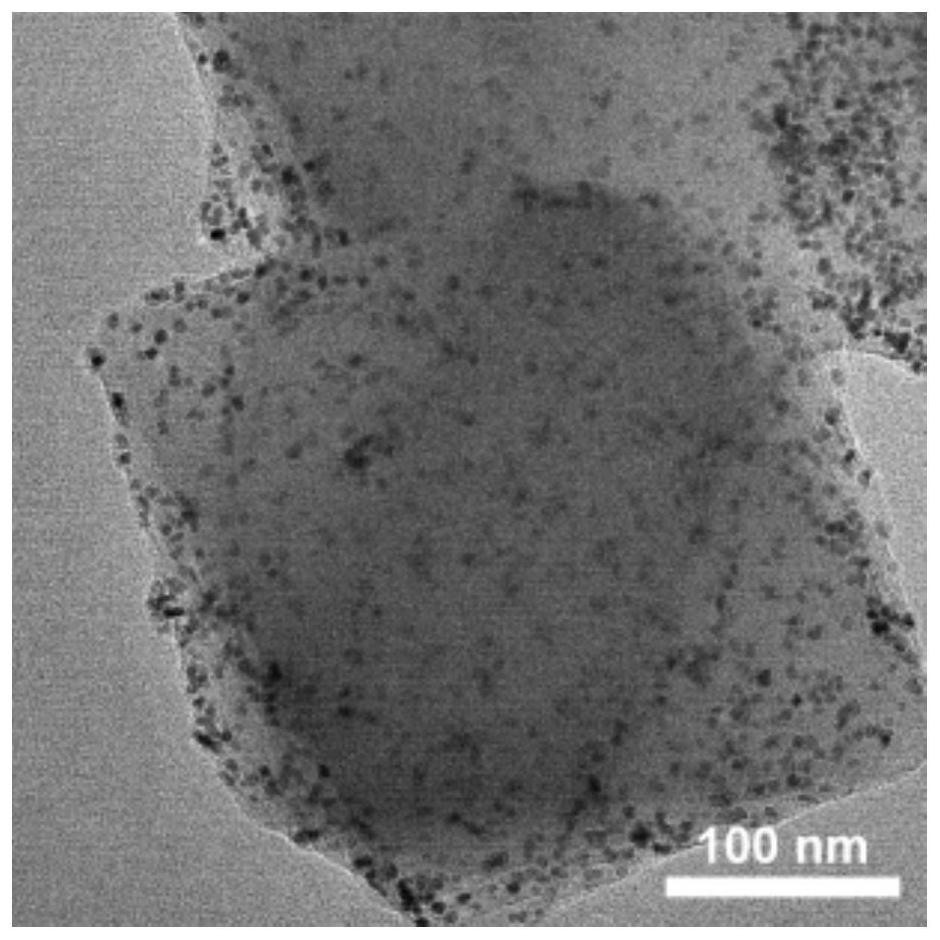
[0070] (2) PtM 2 / UiO-66-NH 2 (M=Ni or Co) preparation: 50mg UiO-66-NH 2 Add it into 5mL DMF, sonicate until uniform dispersion (360W power, 5 minutes sonication), stir at room temperature for 30min to obtain solution A; then add 0.01mmol platinum acetylacetonate and 18mg terephthalic acid to solution A, stir at room temperature for 10min Obtain a uniform solution B; then add 0.02mmol of nickel acetylacetonate or cobalt acetylacetonate to solution B, continue to stir at room temperature for 30min, and keep stirring the resulting solution at 150°C for 12h at a stirring speed of 600rpm. After the reaction, mix The solution was centrifuged at a speed of 8000 rpm, washed twice with DMF and ethanol after removing the supernatant, and finally dried under vacuum at 60°C overnight to obtain PtNi 2 / UiO-66-NH 2 and PtCo 2 / UiO-66-NH 2 .
[007...
Embodiment 3
[0072] Embodiment 3 prepares PtFe 2 / UiO-66 and PtFe 2 / MIL-101(Cr) catalyst
[0073] (1) Preparation of UiO-66: Add 52mg of zirconium tetrachloride and 36mg of terephthalic acid to 50mL of DMF, then add 6mL of glacial acetic acid, stir at room temperature for 30min, transfer the mixed solution to the liner of the polytetrafluoroethylene reactor , then put the liner of the polytetrafluoroethylene reactor into the high-pressure reactor, raise the temperature to 120°C, the solvent volatilizes to form a high-pressure environment, and then react for 12 hours under the condition of high temperature and high pressure at 120°C, and the obtained mixture is heated at 8000 rpm Centrifuge at a high speed, remove the supernatant, wash twice with DMF and absolute ethanol, and finally vacuum-dry at 120°C to obtain UiO-66.
[0074] (2) Preparation of MIL-101 (Cr): Disperse 266.5mg of chromium trichloride hexahydrate and 166.1mg of terephthalic acid into 7.2ml of water, stir vigorously at r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com