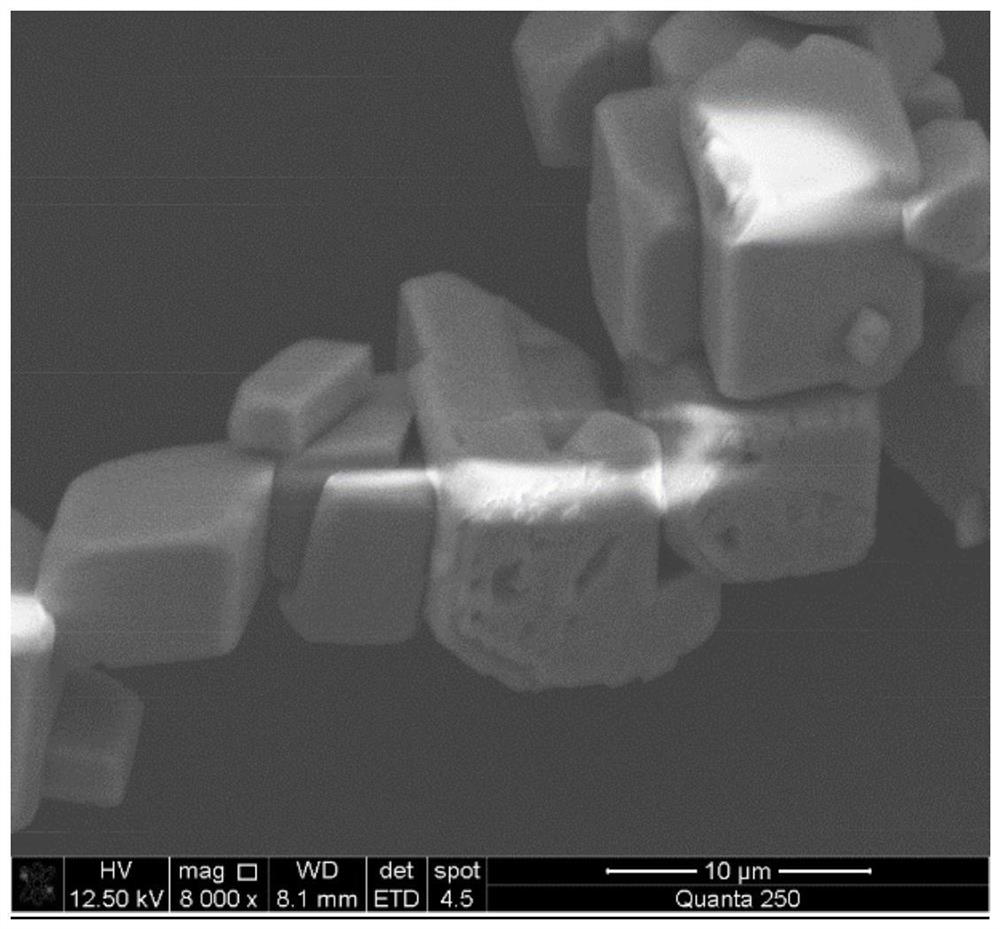
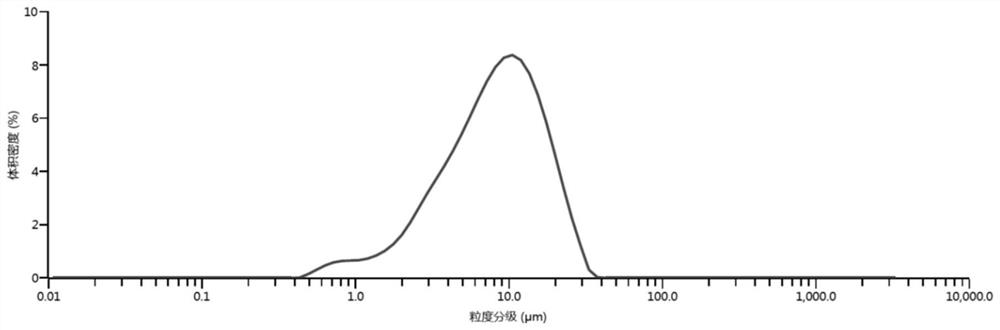
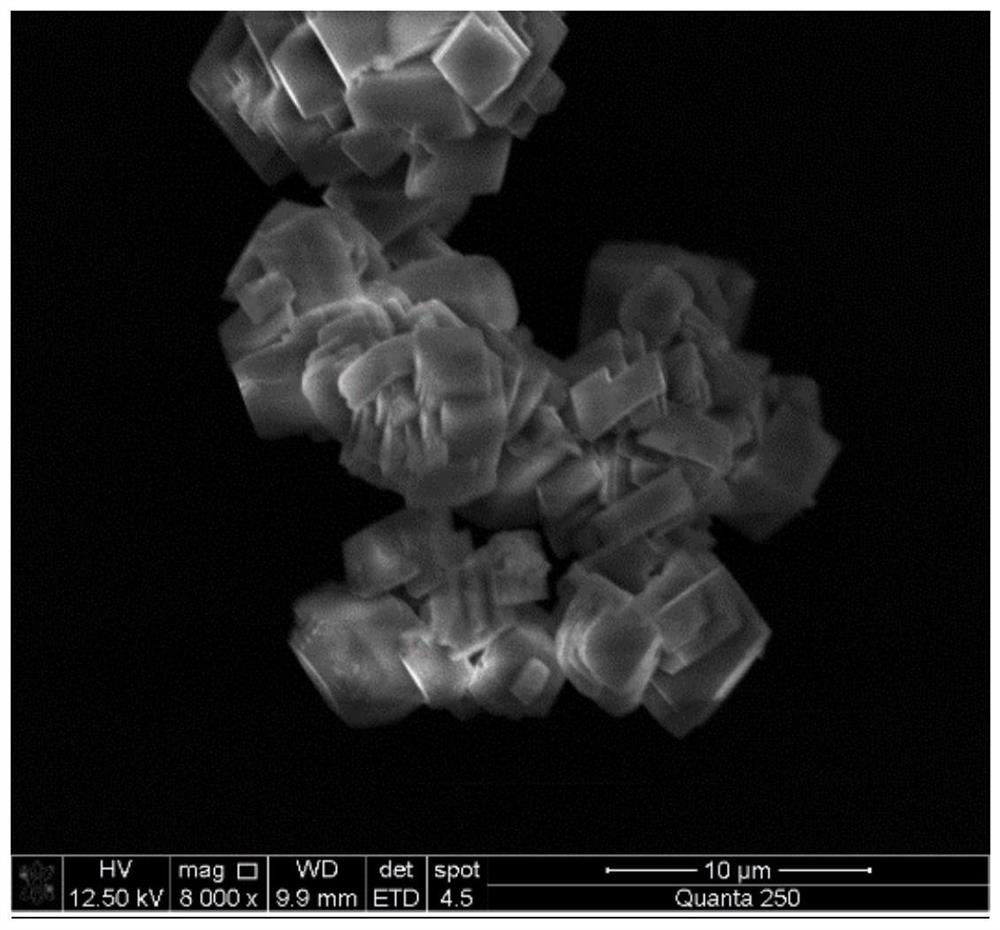
Method for preparing calcium carbonate and hydrogen chloride gas by mineralizing alkali-making distilled ammonia waste liquid
A technology of hydrogen chloride gas and ammonia distillation waste liquid, applied in the directions of chlorine/hydrogen chloride, calcium carbonate/strontium/barium, etc., can solve the problems of wasting resources and harming the environment, and achieves the realization of recycling, reliable and stable operation, and simple process flow. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Calcium carbonate and hydrogen chloride gas are prepared by comprehensively utilizing the ammonia distillation waste liquid and carbon dioxide in the above method. Specifically:
[0044] Get 250ml containing 1mol / L Calcium Chloride Ammonia distillation waste liquid, 80ml industrial tertiary amine N235 as extraction agent, 120ml isoamyl alcohol as diluent in jacketed reactor, control stirring speed to be 200r / min, the The reactants were mixed evenly. The reaction temperature was controlled at 25° C., and carbon dioxide gas was introduced into the reaction system.
[0045] Depending on the progress of the reaction, different intake rates are controlled. Within 1 hour of the reaction, the intake rate is controlled to 50ml / min; The air intake rate was 5ml / min, and the reaction was stopped after 3.5 hours. The calcium carbonate powder was obtained by filtering solid-liquid separation, washed alternately with deionized water to remove entrained organic impurities and water...
Embodiment 2
[0048]Calcium carbonate and hydrogen chloride gas are prepared by comprehensively utilizing the ammonia distillation waste liquid and carbon dioxide in the above method. Specifically:
[0049] Take 150ml of alkali-making ammonia distillation waste liquid containing 1.5mol / L calcium chloride, 250ml of primary amine N1923 as extractant, 125ml of isopropanol, and 250ml of isoamyl alcohol as diluent in a jacketed reactor, and control the stirring speed to 300r / min to mix the reactants evenly. The reaction temperature was controlled at 30° C., and carbon dioxide gas was introduced into the reaction system.
[0050] According to the different progress of the reaction, different intake rates are controlled. Within 1 hour of the reaction, the intake rate is controlled to 55ml / min; The gas rate was 5ml / min, and the reaction was stopped after 3 hours. Through solid-liquid separation by filtration, calcium carbonate powder was obtained, washed alternately with deionized water to remo...
Embodiment 3
[0053] Calcium carbonate and hydrogen chloride gas are prepared by comprehensively utilizing the ammonia distillation waste liquid and carbon dioxide in the above method. Specifically:
[0054] Get 150ml containing 2mol / L Calcium Chloride Ammonia distillation waste liquid, 125ml industrial tertiary amine N235 as extractant and 125ml methyl isobutyl ketone as diluent in jacketed reactor, control stirring speed is 200r / min, The reactants were mixed well. The reaction temperature was controlled at 10° C., and carbon dioxide gas was introduced into the reaction system.
[0055] According to the different progress of the reaction, different intake rates are controlled. Within 1 hour of the reaction, the intake rate is controlled to 80ml / min; The gas rate was 6ml / min, and the reaction was stopped after 3 hours. Through solid-liquid separation by filtration, calcium carbonate powder was obtained, washed alternately with deionized water to remove entrained organic impurities and wa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com