Preparation method of tert-octylamine
A technology of tert-octylamine and tert-octylacetamide, which is applied in the field of preparation of tert-octylamine, can solve the problems of sensitive acylase reaction conditions, complicated hydrolysis process of acetamide, and low total yield of tert-octylamine, and achieves raw material Easy to obtain, increase yield and reduce production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
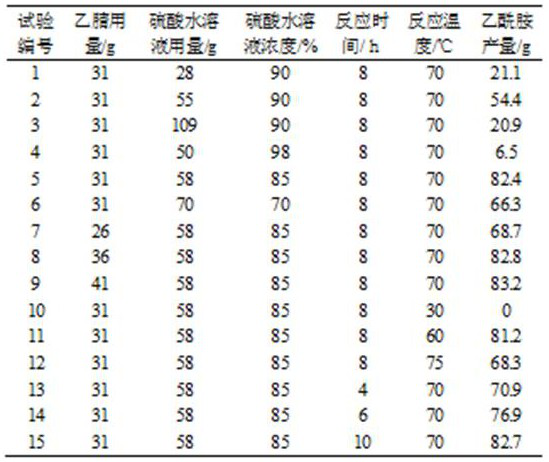
[0028] The mixture was stirred, 56 g of diisobutylene, 31 g of acetonitrile, 55 g concentration of 90% aqueous sulfuric acid solution and 1 g of phase transfer catalyst were added to the round bottom flask, and an amidated reaction was carried out at 70 ° C for 8 h. The reaction liquid recovered the phase transfer catalyst and the unreacted acetonitrile, diisobutylene, after water, added to a certain amount of an alkaline solution, and adjusted the solution pH microbialism, filtered, washed to the filtrate, neutral N-coushanine Acetamide contains water filter cake. The filter cake is dried in a vacuum oven to a vacuum oven to a constant weight, and the reserved sealing storage is obtained.
[0029] Effect of Table 1 Different phase transfer catalysts on the production of acetamide
[0030]
Embodiment 2
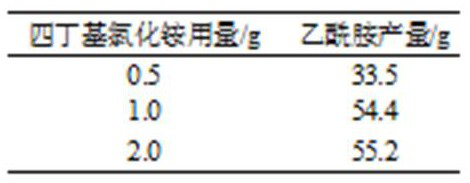
[0032] The mixture was stirred, 56 g of diusbutene, 31 g of acetonitrile, 55 g of a 20% aqueous solution of sulfuric acid and a certain amount of tetrabutylammonium chloride were added to the round bottom flask, and an amidation reaction was carried out at 70 ° C for 8 h. The reaction solution recovered tetrabutylammonium chloride and unreacted acetonitrile, diisobutylene, water was added to a certain amount of an alkaline solution, and after adjusting the pH of the solution, filtered, washed to the filtrate, neutral N- Uttal acetamide contains water filter cake. The filter cake is dried in a vacuum oven to a vacuum oven to a constant weight, and the reserved sealing storage is obtained.
[0033] Effect of Table 2 Phase Transfer Catalyst Dosage on Acetamide Yield
[0034]
Embodiment 3
[0036] The mixture was stirred, 56 g of diisobutylene, acetonitrile, sulfuric acid solution, and 1 g of tetrabutylammonium chloride were added to the round bottom flask for an amidation reaction. At the end of the reaction, tetrabutylammonium chloride and an unreacted acetonitrile, diisobutylene, after water, the reaction solution was added to a certain amount of an alkaline solution, and the solution pH is subjected to the solution, filtered, washed to the filtrate. N-tertianedon-containing acetamide containing water filter cake. The filter cake is dried in a vacuum oven to a vacuum oven to a constant weight, and the reserved sealing storage is obtained.
[0037] Table 3 Optimization of acetamide reaction process parameters
[0038]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com