Isosteviol derivative as well as preparation method and application thereof
A technology of isosteviol and its derivatives, which is applied in the field of isosteviol derivatives and its preparation, can solve the problems of high cardiovascular risk and achieve good cardioprotective activity and the effect of protecting death and injury
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] This example provides a series of isosteviol derivatives, prepared according to the following two synthetic routes:
[0055] Synthetic route one:
[0056] Reaction conditions and reagents: (a) NaOH, (HCHO)n , EtOH, reflux; (b) Ac 2 O, Pyridine, DMAP for 3a; (c) Acid, DMAP, EDCI, DCM for 3b-3n; (d) PDC, DMF, rt.)
[0057]
[0058] Synthetic route two:
[0059] Reaction conditions and reagents: (a) DPPA, Et 3 N, t BuOH, reflux; (b) Acid, DMAP, EDCI, DCM; (c) PDC, DMF.
[0060]
[0061] Specifically, the preparation method of each compound comprises the following steps:
[0062] (1) Synthesis of Compound 2
[0063] Isosteviol (50mg, 0.2mmol), sodium hydroxide (31.4mg, 0.8mmol) and paraformaldehyde (37.8mg, 1.3mmol) were dissolved in absolute ethanol (2mL), the mixture was stirred at 80°C, and the reaction 5h. The mixture was diluted with ethyl acetate, neutralized with hydrochloric acid (1N), the solution was washed with saturated brine, dried over anhydrous ...
Embodiment 2
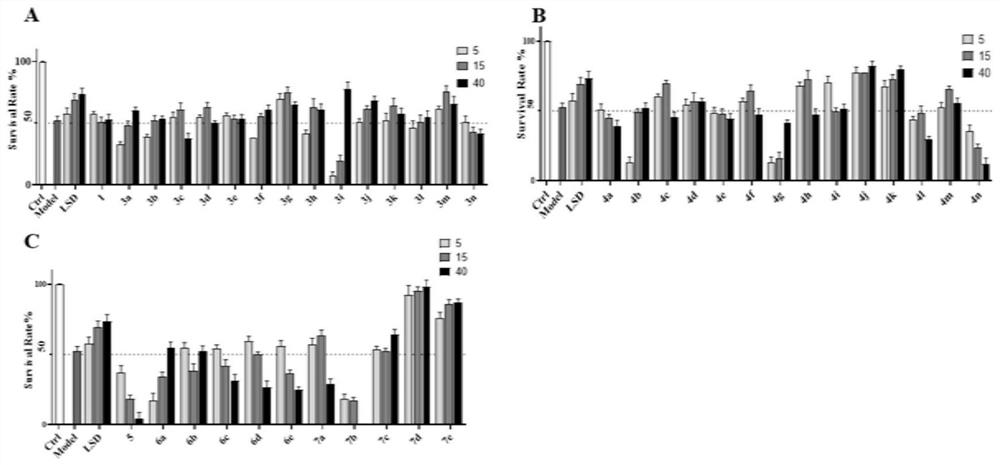
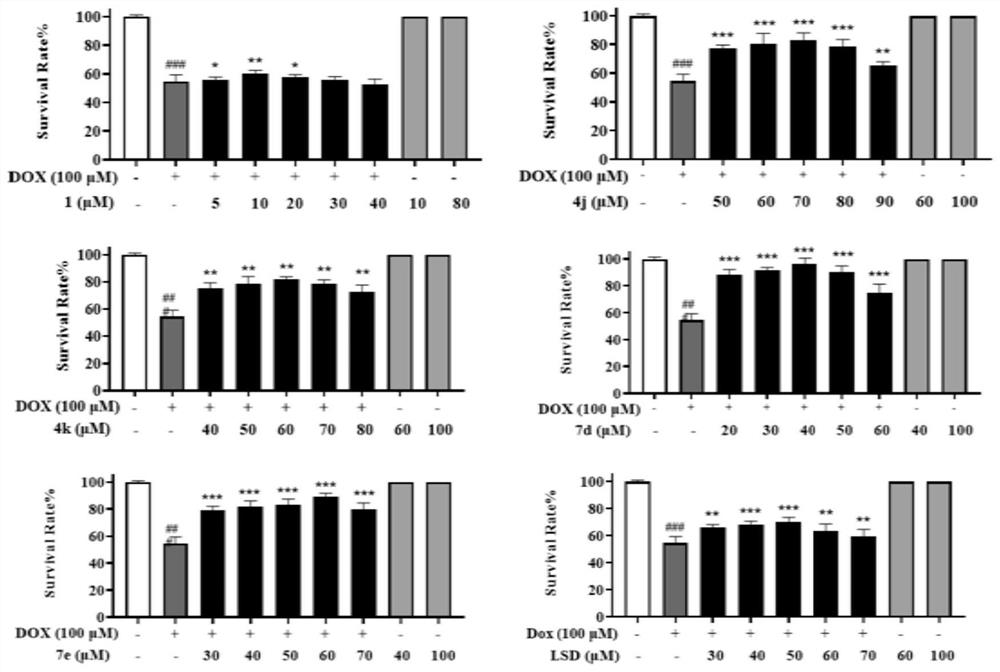
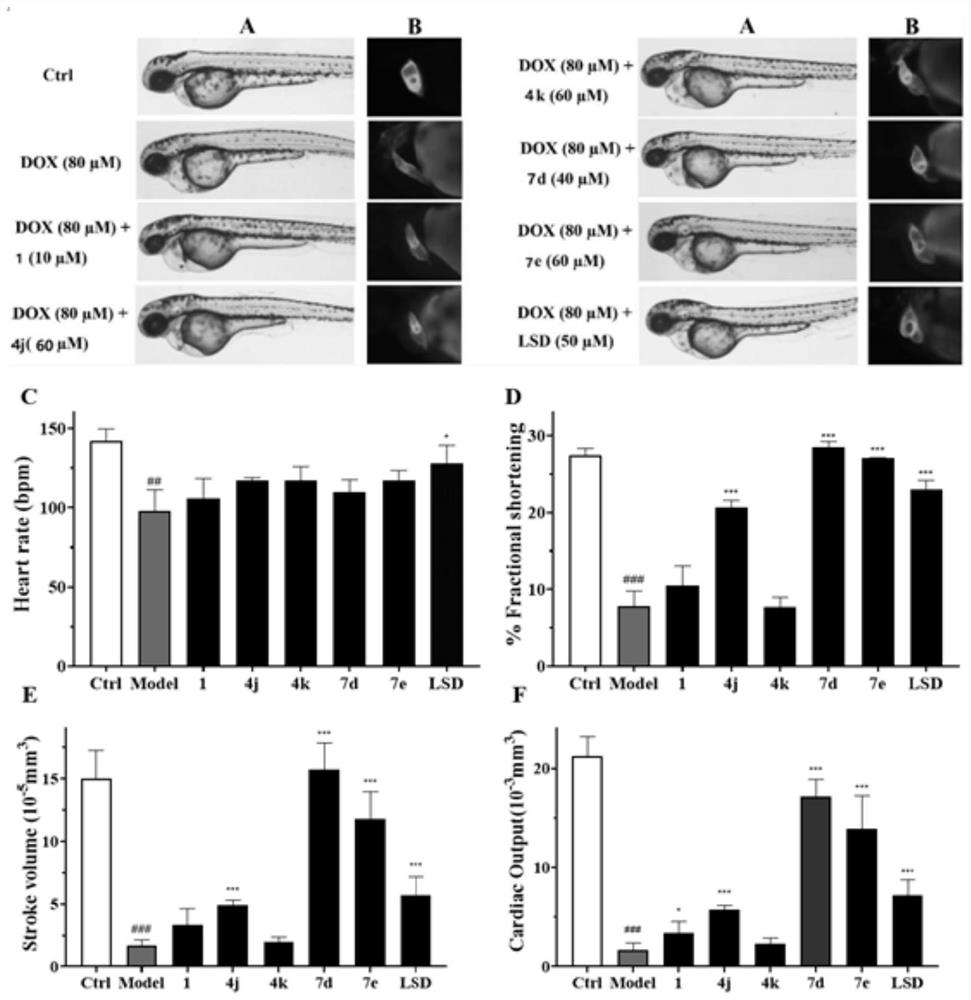
[0195] This example is a zebrafish experiment on a series of isosteviol derivatives prepared in the example, and the cardiac activity of the isosteviol derivatives of the present invention is evaluated through the zebrafish experiment.
[0196] (1) Breeding of zebrafish and collection of embryos: buy zebrafish (3-12 months old) from the National Zebrafish Resource Center, and raise them in a mobile culture with a photoperiod of 14:10h (light: dark) and a temperature of 28.5±1°C Breeding in the box, feeding with live brine shrimp twice a day; mating of zebrafish (female:male=1:1), collecting embryos, washing with Holt Buffer buffer, and culturing in an incubator (28.5±1°C) for 24h , screened by microscopic examination.
[0197](2) DOX-induced zebrafish embryonic heart failure model for drug screening: 24hpf wild-type zebrafish embryos were allocated to 24-well plates (20 embryos / well), and 1 mL of DOX (100 μM) and various test compounds (5 , 15, 40μM), treated for 72h, observe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com