Application of selenium preparation in preparation of medicine for treating Crohn disease
A Crohn's disease and preparation technology, which is applied to the application field of selenium preparations in the preparation of medicines for treating Crohn's disease, can solve the problems such as the treatment effect of selenium supplement preparations that have not been disclosed in the literature, and achieve the improvement of enteritis symptoms and the promotion of remission. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
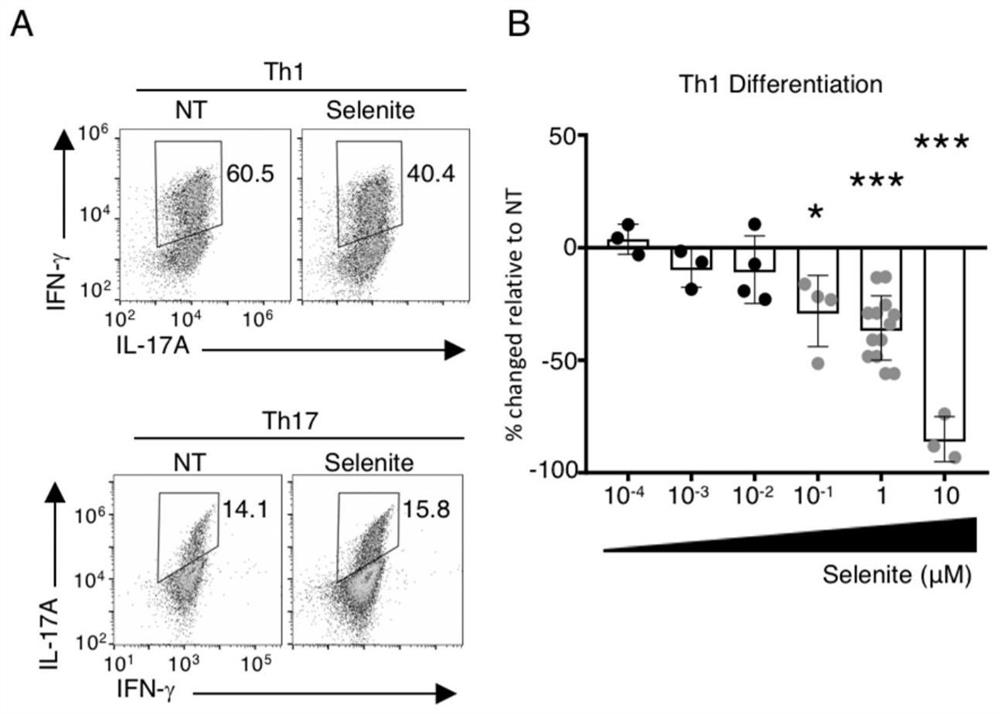
[0023] 1 in vitro cell experimental implementation
[0024] 1.1 Mouse initial CD4 + T cell acquisition:
[0025] The mice were separated from the cervical spot, separated from the underarm, the mandibular lymph nodes and spleen, treated with a syringe to ground lymph nodes and spleen, filtrate, centrifugal, 180 ul rpmi + 20 μL anti-mouse CD4 magnetic bead, 4 ° C incubation 15 Minutes; 2 ml of washing; CD4 + T cells were selected by the LS column. The collected cell fluid was resuspended with 100 μl of 10% FBS RPMI, and antibody FITC ANTI-MOUSE CD44 was added, 1 μl of APC Anti-Mouse Cd62L was added to 4 ° C. Add 10% FBS RPMi2ML to centrifuge cells, discard it, resuspended to 600 μL, sorted by Arial II stream sorter, obtained CD44 lo CD62L hi The population is initial CD4 + T cell.
[0026] 1.2 T cell differentiation experiment:
[0027] The Anti-CD3 / Anti-CD28 package was 24-well plate, 4 ° C overnight. The initial T cells obtained according to the above steps are 4 × 10 5 The wel...
Embodiment 2
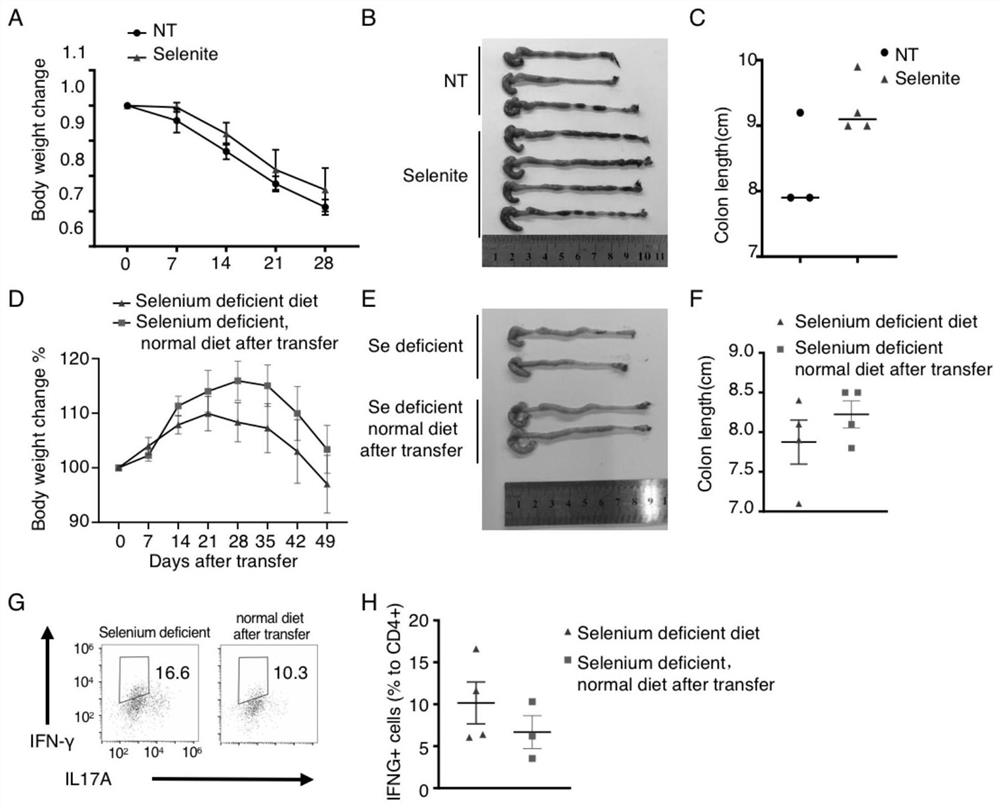
[0031] 2 animal enteritis model implementation
[0032] 2.1 experiment group
[0033] Piece method 1: 1 control group (normal diet drinking water); 2 selenium group (0.8 ppm of sodium selenite in drinking water).
[0034] Group 2: RAG1 - / - Mouse fed selenium feed (0 ppm) 4-8 weeks, establish a selenium in vivo, and then divided into two groups, 1 group continued to maintain selenium feed to the end of manufacturing, 2 other colitis model After the addition of selenium diet (0.2 ppm) and sodium selenate (0.8 ppm).
[0035] 2.2 Experimental Process
[0036] CD4 of a wild-type mouse by using a magnetic bead sorting a streaming cytometry + CD45RB hi T cells, end-tail intravenous injection RAG1 - / - Mouse (5 × 10 5 / only). Weigh the weight per week, and complete the model after 6-8 weeks. Mouse weight, statistical mouse mortality, etc. during modeling. After the mold, mice were sacrificed, and the length of the intestine was measured, and the tissue specimens at the anus 1, 3, 8 cm, and...
Embodiment approach
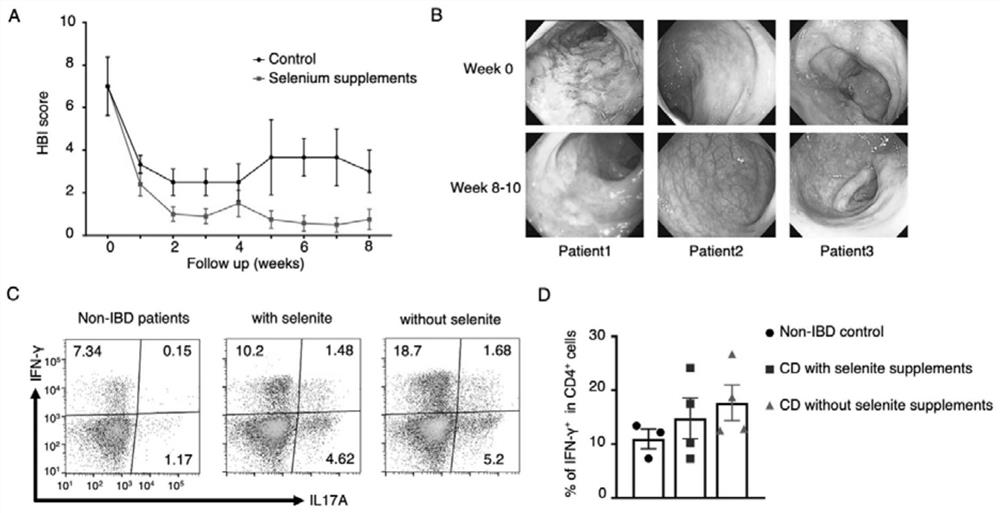
[0041] 1 Study population:
[0042] standard constrain
[0043] 1 age 18-60 years old;
[0044] 2 Combined with medical history, physical examination, auxiliary examination, etc. confirmed the diagnosis of Coron disease patients.
[0045] 3 Agree to detect the peripheral blood selenium level, and the selenium concentration is less than 84μg / L
[0046] 4 Agree to participate in this study and sign this research informed consent
[0047] Exclude standard:
[0048] 1 The combined person suffers from any unstable or uncontrolled cardiovascular, lung, liver, kidney, gastrointestinal tract, urinary hematology, coagulation function, immunization, endocrine / metabolism or other medical diseases, and researchers believe that the results of the interference Or endanger the safety of the subject.
[0049] 2 The results of lactating female subjects or serum pregnancy test results are positive.
[0050] 3.2 Administration / Treatment / Observation Program:
[0051] Serum selenium concentrat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com