Method for preparing pyridotriazolone compounds by flow chemistry
A technology of pyridotriazolone and flow chemistry, which is applied in the fields of organic chemistry, chemical industry, sustainable manufacturing/processing, etc., can solve the problem that triazolone cannot be synthesized, achieve less back-mixing, high mass transfer and heat transfer efficiency , reduce the effect of side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025]
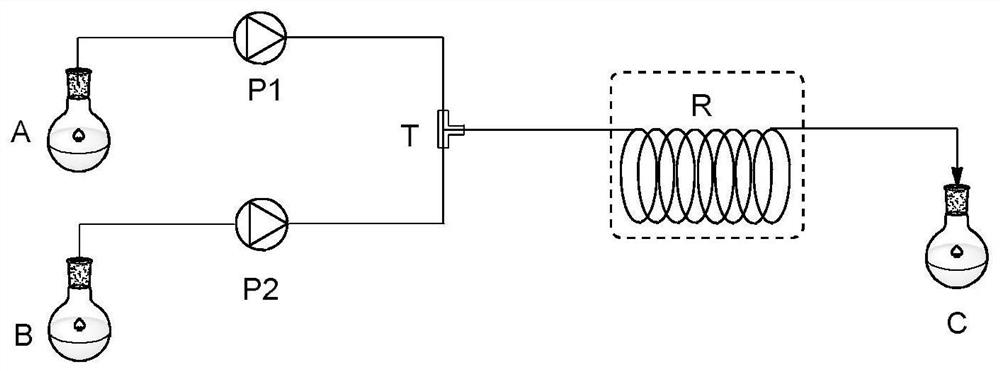
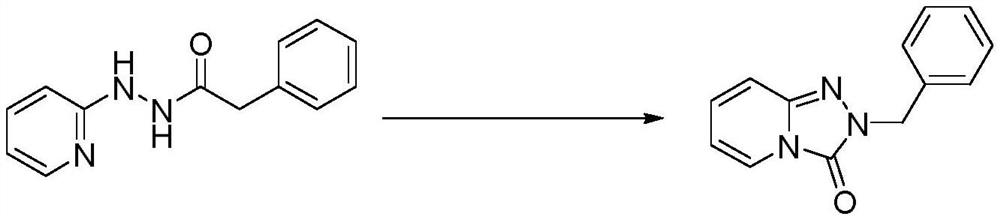
[0026] Add the raw material 2-phenyl-N'-(pyridin-2-yl)acetylhydrazide (30mmol) into the first storage tank, dissolve it with methanol until it is clear and set the volume to 60mL, and prepare a hydrazide concentration of 0.5mmol / mL solution, stir well before use. Add iodobenzene diacetate (33mmol) into the second storage tank, dissolve it with methanol until clarification and set volume to 66mL to prepare a solution with an oxidant concentration of 0.5mmol / mL, stir well and set aside for use.
[0027] The materials in the first storage tank and the second storage tank are conveyed by metering pumps P1 and P2 respectively (the molar flow ratio of raw materials and oxidants is 1:1.1), enter the mixer, and mix, and the mixed reaction liquid enters the tubular reaction The reactor was oxidized and rearranged, the temperature of the tubular reactor was maintained at 45°C, and the residence time of the reaction liquid in the tubular reactor was 30 minutes. The reaction ...
Embodiment 2
[0029] According to the method described in Example 1, except that acetonitrile was used instead of methanol as the solvent, the yield of the product was 31%, and the product was a white solid.
Embodiment 3
[0031] According to the method described in Example 1, except that the reaction temperature was 70° C., the yield of the product was 81%, and the product was a white solid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com