Dimethylamine sphaelactone-p-hydroxybenzoate as well as preparation method and application thereof
A technology of hydroxybenzoate and p-hydroxybenzoic acid, which is applied in the field of dimethylamine lagalactone-p-hydroxybenzoate and preparation, can solve the problems of poor controllability of technological process and product quality, and easy interaction of crystal forms. Transformation, complex preparation process and other problems, to achieve the effect of convenient industrialized large-scale production and application, easy storage and transportation, and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Add 1.5g of dimethylamine michelactone solid, 1.414g of p-hydroxybenzoic acid solid, and 75mL of isopropyl acetate into the reaction flask, heat to 60°C, and stir for 30 minutes to fully react the raw materials. Cool to 0°C at a rate of 5°C / h, filter the obtained product, and then dry under normal pressure at 40°C for 3 hours to obtain dimethylaminomildolide-p-hydroxybenzoate.
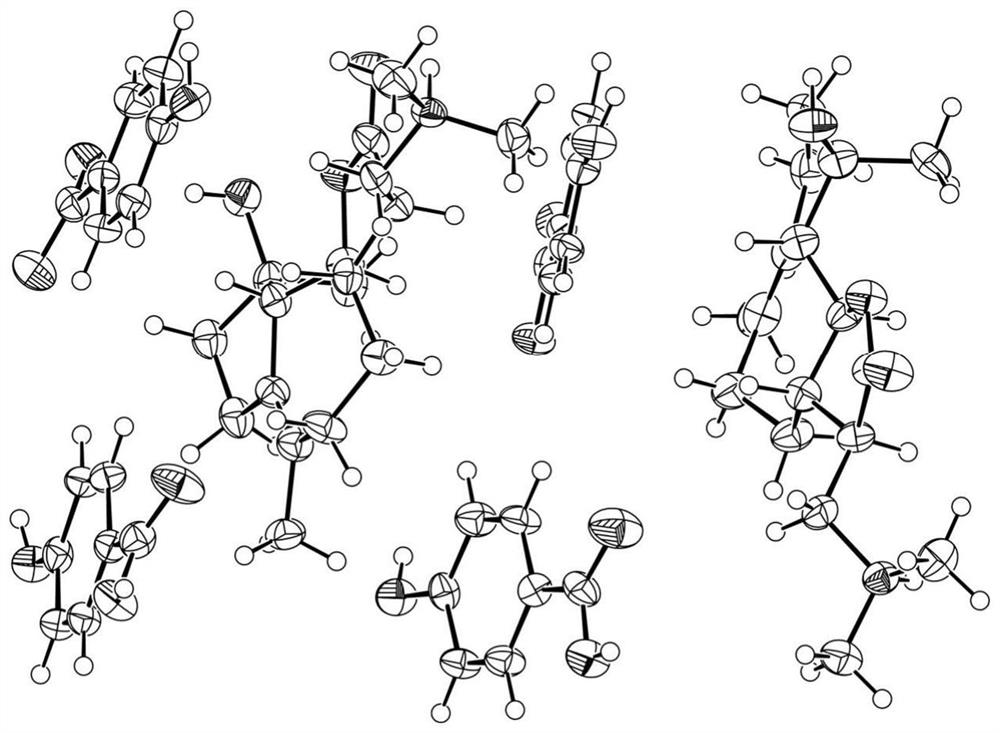
[0061] figure 1 Prepare the crystallographic characteristics of the product for embodiment 1, from figure 1 It can be seen that the crystallographic characteristics are: the space group is P2 1 2 1 2 1 , the cell parameters are α=90°, β=90°, γ=90°, the unit cell volume is
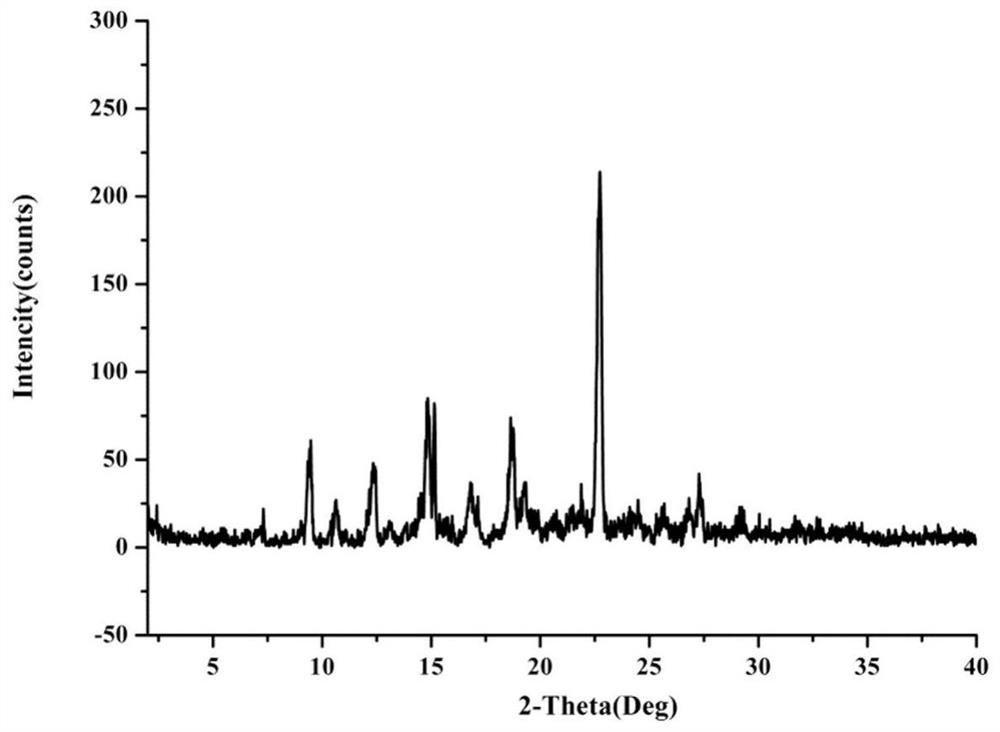
[0062] figure 2 Prepare the powder X-ray diffraction pattern of product for embodiment 1, from figure 2 It can be seen that the diffraction angle is 2θ at 5.5°, 7.3°, 9.5°, 10.7°, 12.4°, 13.2°, 13.9°, 14.9°, 16.9°, 17.2°, 18.8°, 19.5°, 20.0°, 20.5° , 21.5°, 22.0°, 22.8°, 24.2°, 25.7°, 27.3°, 29.3° have characte...
Embodiment 2
[0091] Add 3 g of dimethylamine michelactone solid, 3.111 g of p-hydroxybenzoic acid solid, and 30 mL of methanol into the reaction flask, heat to 55°C, and stir for 10 min to fully react the raw materials. Cool to -10°C at a rate of 2°C / h, filter the obtained product, and then dry under normal pressure at 30°C for 2 hours to obtain dimethylaminomildolide-p-hydroxybenzoate.
[0092] The product that embodiment 2 obtains is carried out crystallographic test, and test result is the same as embodiment 1.
[0093] Carry out PXRD test to the product that embodiment 2 obtains, as can be known: with diffraction angle being 2θ, represent at 5.6 °, 7.5 °, 9.7 °, 10.8 °, 12.5 °, 13.4 °, 13.8 °, 14.9 °, 16.8 °, 17.2 °, There are characteristic peaks at 18.8°, 19.6°, 20.1°, 20.6°, 21.7°, 22.2°, 22.9°, 24.4°, 25.9°, 27.5°, 29.4°.
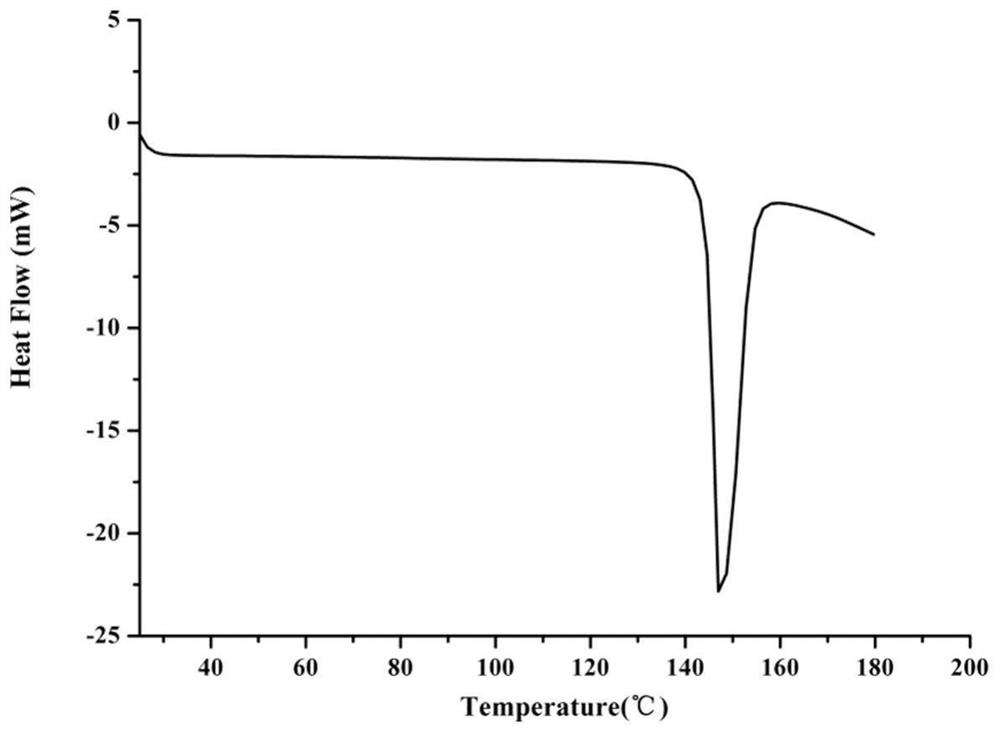
[0094] Carry out DSC test to embodiment 2, know: product has endothermic peak at 145.2 ℃.
[0095] Adopt the method identical with embodiment 1 to carry out f...
Embodiment 3
[0110] Add 6g of dimethylamine michelactone solid, 7.065g of p-hydroxybenzoic acid solid, and 120mL of acetone into the reaction flask, heat to 45°C, and stir for 20min to fully react the raw materials. Cool to 15°C at a rate of 3°C / h, filter the obtained product, and then dry under normal pressure at 25°C for 3 hours to obtain dimethylaminomildolide-p-hydroxybenzoate.
[0111] The product that embodiment 3 obtains is carried out crystallographic test, and test result is the same as embodiment 1.
[0112] Carry out PXRD test to the product that embodiment 3 obtains, as can be known: with diffraction angle being 2θ, represent at 5.3 °, 7.1 °, 9.3 °, 10.5 °, 12.2 °, 13.0 °, 13.7 °, 14.7 °, 16.8 °, 17.1 °, There are characteristic peaks at 18.6°, 19.3°, 20.1°, 20.3°, 21.3°, 21.9°, 22.6°, 24.0°, 25.5°, 27.1°, 29.1°.
[0113] Carry out DSC test to embodiment 3, know: product has endothermic peak at 145.5 ℃.
[0114] Adopt the method identical with embodiment 1 to carry out follow...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com