Hydrogen chloride oxidation reaction catalyst for preparing chlorine, and preparation method therefor
A catalyst, hydrogen chloride technology, applied in the direction of chloride preparation, metal/metal oxide/metal hydroxide catalyst, catalyst activation/preparation, etc., can solve the problems of reduced catalytic performance and low thermal stability, and achieve strengthening Thermal stability, ensuring thermal stability, and the effect of maintaining catalytic performance for a long time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0053] According to an embodiment of the present invention, a method for preparing a catalyst for hydrogen chloride oxidation reaction is provided, comprising the following steps: Step (a), the first loading step, preparing more than one precursor selected from heterogeneous substances and dissolving them in a solvent The resulting solution is loaded onto one or more carriers selected from alumina, titania and zirconia; step (b), after the first loading step, is dried and fired, and cooled at room temperature , to obtain a solid; step (c), the second loading step, prepare a solution in which the ruthenium precursor is dissolved in a solvent, and further carry out loading; step (d), after the second loading step, drop into the solids; and step (e), performing a second drying and firing.
[0054] At this time, the foreign substances include more than one selected from cerium precursors, aluminum precursors, and silicon dioxide precursors, and relative to the solution with a tota...
Embodiment 1
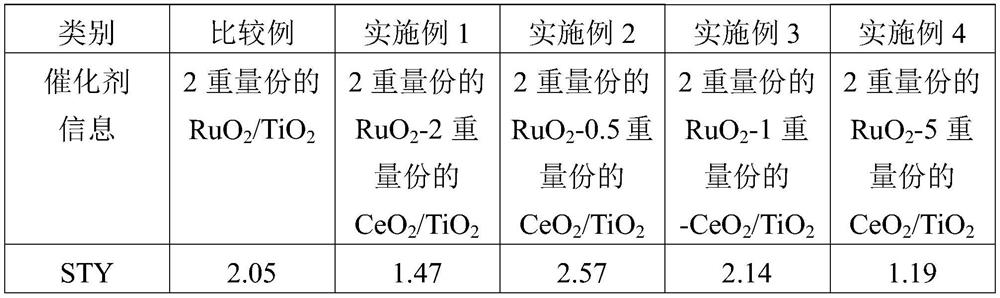
[0073] A solution prepared by dissolving 0.5 g of cerium nitrate hydrate in 5.0 g of DIW was impregnated with 10.0 g of titanium dioxide powder, and then dried in air at 100° C. for 4 hours. The dried solid was subjected to calcination at 350° C. for 3 hours in an electric furnace with air flow, after which it was slowly cooled to room temperature. In the solution prepared by dissolving 1.08 g of trinitronitrosyl ruthenium dissolved in nitric acid solution in 320.0 g of DIW, add the solid matter prepared in the above-mentioned way, and stir at room temperature for 5 hours, after that, use rotation Concentrator for drying. In an electric furnace with air flow, at 350 ° C, the dried solid was calcined for 3 hours, and then slowly cooled to room temperature, finally, the content of ruthenium oxide was 2.0 parts by weight. The content of cerium is 2.0 parts by weight of RuO 2 -CeO 2 / TiO 2 catalyst.
Embodiment 2
[0075] Using 0.13 g of cerium nitrate hydrate, the catalyst was prepared in the same manner as in Example 1. Finally, the RuO with a content of ruthenium oxide of 2.0 parts by weight and a content of cerium oxide of 0.5 parts by weight was obtained. 2 -CeO 2 / TiO 2 catalyst.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com