Improved rechargeable batteries and production thereof
A battery cell and electrolyte technology, applied in the field of rechargeable electrochemical battery cells, can solve problems such as high flammability, low chemical stability of electrolyte salt, and limited battery operating temperature range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
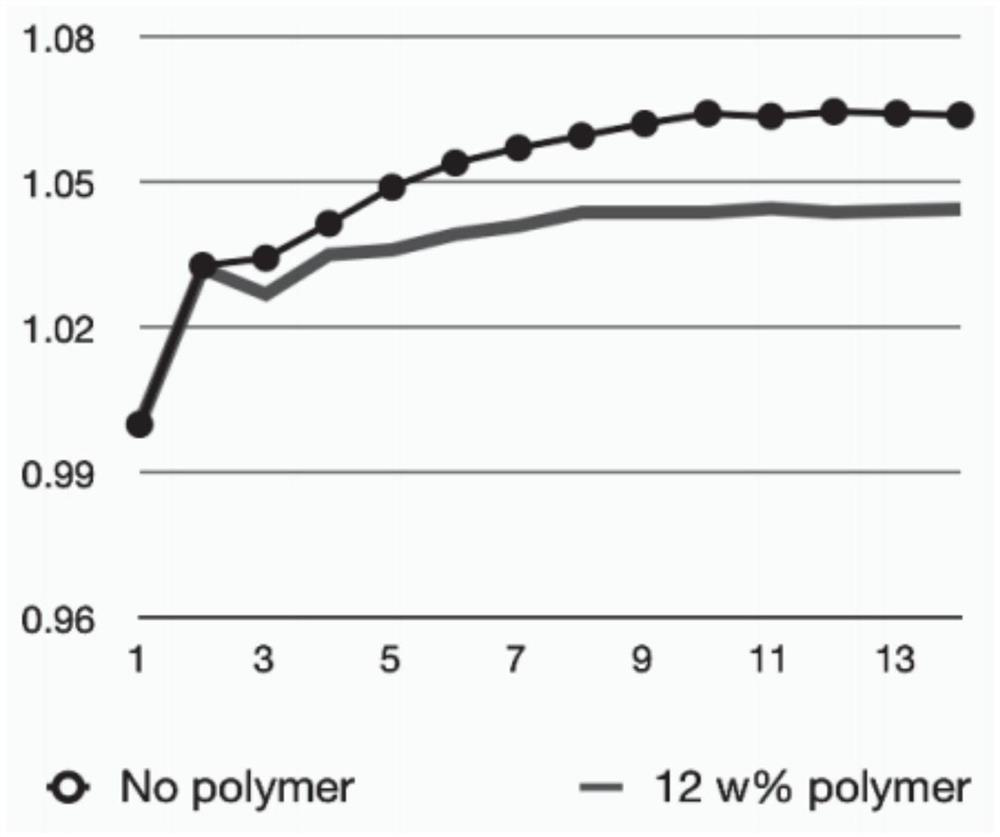
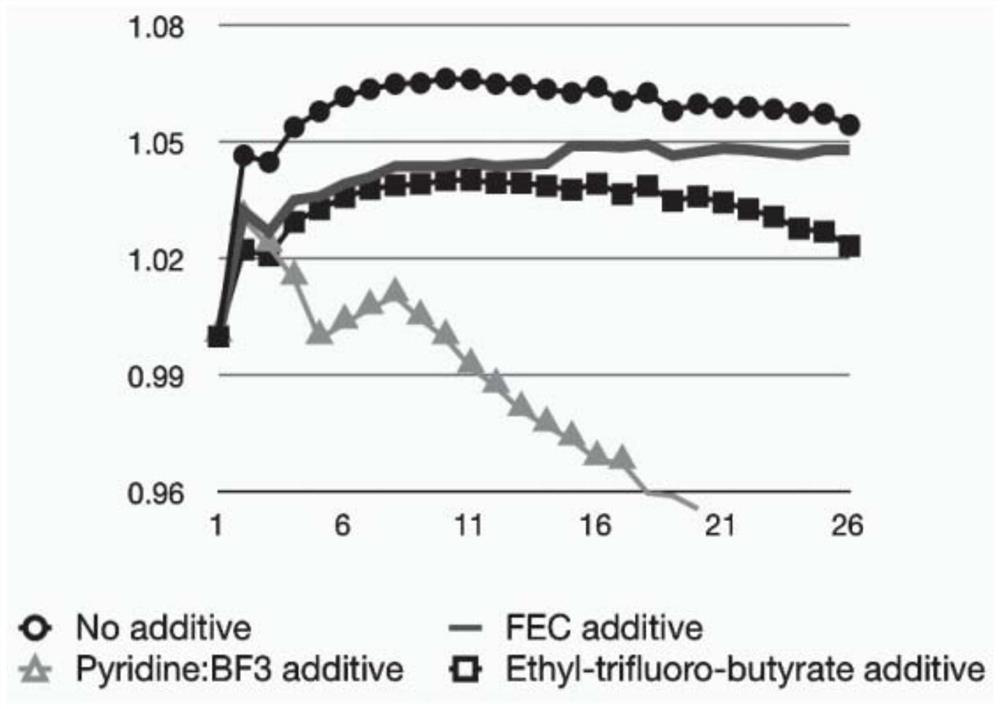
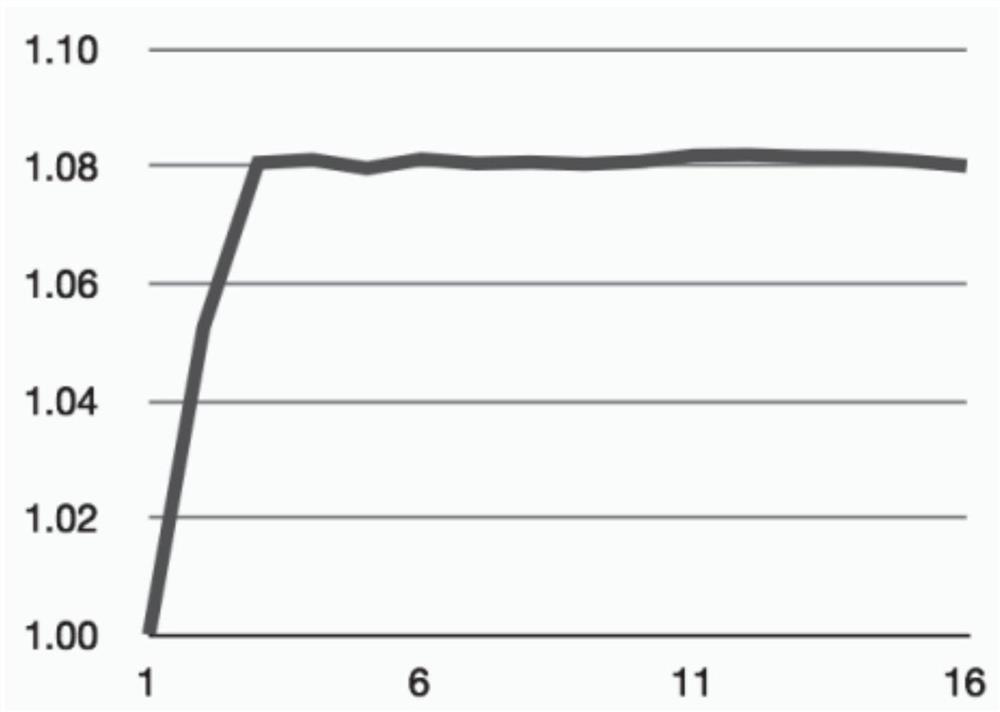
[0029] LiMn x Fe 1-x PO 4 (LMFP for short, also known as LFMP) is a cobalt-free battery cathode material that has attracted much attention in recent years. We tested the cycling stability of the LMFP cathode at a charging voltage of 4.2 V and investigated the effect of electrolyte additives. figure 2 The cathodic stability of the LMFP electrode in different electrolyte variants is shown, and in particular the discharge capacity evolution of the LMFP cathode with respect to the number of charge-discharge cycles is shown. The scale is normalized to the initial discharge capacity of each electrode. In all cases, the initial gravimetric discharge capacity was about 150 mAh / g relative to the LMFP weight. The electrolyte solvent comprises DMC:SCN with a volume ratio of 1:1, further comprising 12% by weight poly(methyl vinyl ether-alanine-maleic anhydride) additive, and further comprising 1 mole of LiDFOB salt. Further electrolyte additives used are shown in the figure. We ha...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com