Gene medicine for treating testicular interstitial cell dysfunction and application thereof
A technology of Leydig cells and dysfunction, which is applied in the field of gene medicine for the treatment of Leydig cell dysfunction, can solve problems such as inability to solve male infertility, and achieve high safety, specificity and safety effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1: Construction and separation and purification of AAV vector
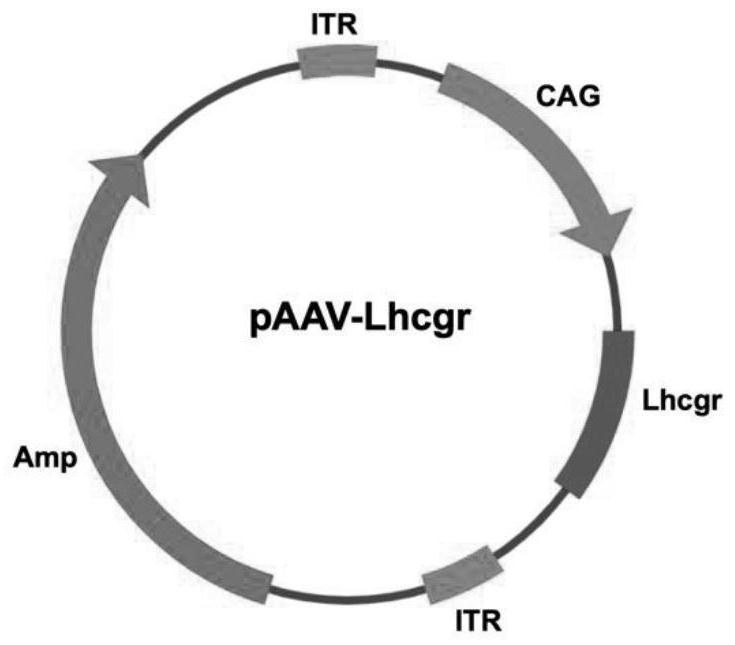
[0039] Plasmid AAV-Lhcgr was constructed as figure 1 As shown, the main elements include CAG promoter and Lhcgr sequence. Viral vectors are obtained by plasmid co-transfection. Different types of AAV Rep / Cap plasmids, AAV-Helper plasmids and AAV-Lhcgr plasmids are co-transfected with HEK 293 cells to form AAV vectors. After purification by iodixanol density gradient ultracentrifugation, the titer of the virus was determined by real-time fluorescent quantitative PCR, and finally the silver staining method was used to confirm that the virus vector particles were not contaminated and did not contain endotoxin, and were divided into -80 Store at ℃.
Embodiment 2
[0040] Example 2: Carrier Screening
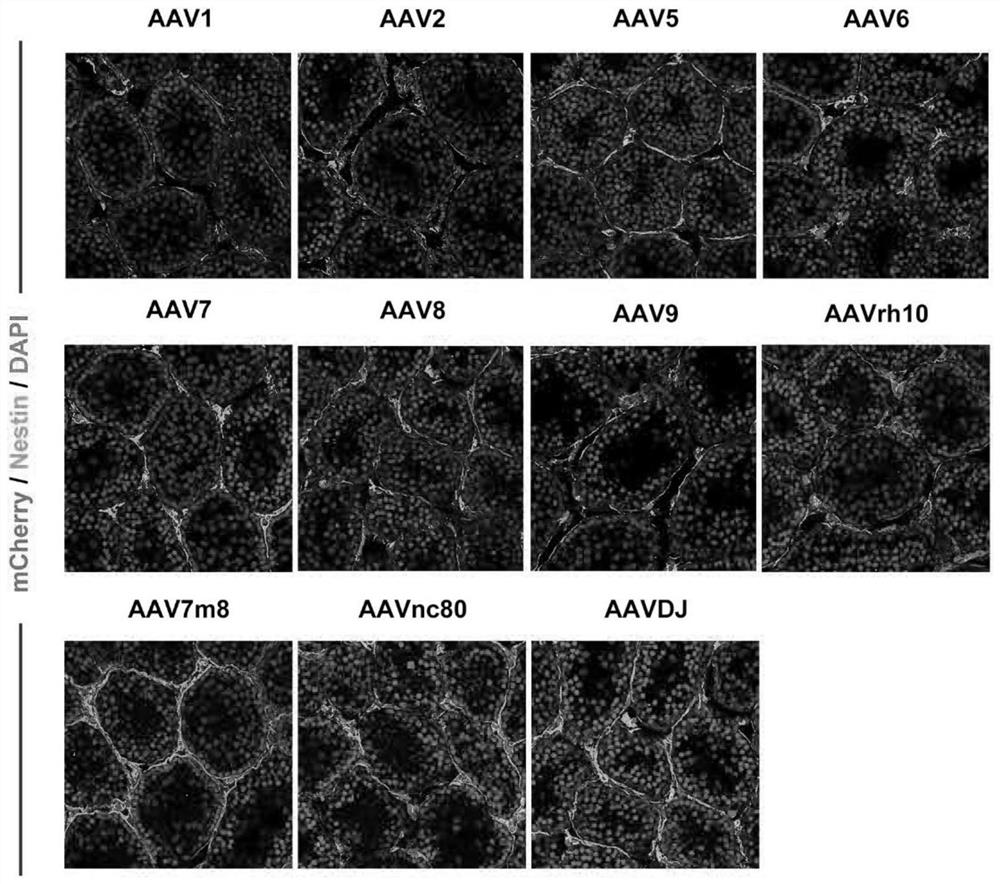
[0041] To construct an AAV vector carrying mCherry, we screened 11 AAV serotypes AAV1, AAV2, AAV5, AAV6, AAV7, AAV8, AAV9, AAVrh10, AAV7m8, AAVnc80, AAVDJ. Dilute the constructed viral vector composition to 1×10 with saline 13 vg / mL, after the mouse was anesthetized with 0.2% avertin (0.2 mL / 10 g), 8 μL of the diluted carrier composition was injected into each testis on both sides with a microinjector. One week later, the mouse testis tissue was taken for tissue immunofluorescence staining, and Leydig cell precursor cell marker Nestin was stained; finally, a confocal microscope was used to observe and take pictures.
[0042] figure 2 It shows that AAV5, AAV6, AAV8, AAV9, AAVrh10, AAVnc80, AAVDJ and Nestin have a higher co-expression ratio, suggesting that the above AAV types can better infect Leydig cell precursor cells.
Embodiment 3
[0043] Example 3: gene drug expresses Lhcgr, restores testosterone level, and promotes the maturation of Leydig cells
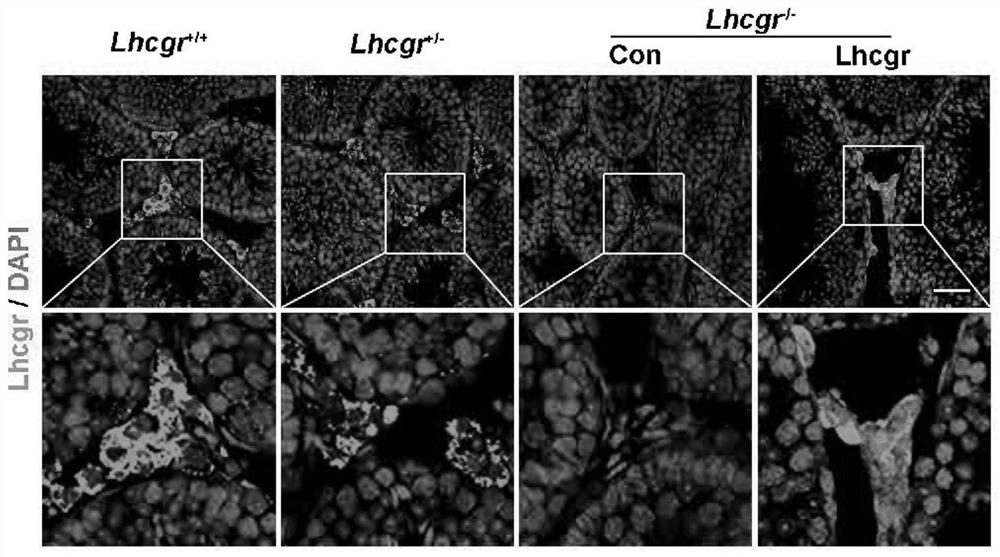
[0044]AAV8 in Example 2 was selected as a gene delivery tool, and an AAV8-CAG-Lhcgr viral vector (gene drug) was constructed. In order to detect whether the gene carrier is successfully expressed and functioning in the testis; 3w-age Lhcgr - / - Mice were injected with AAV8-Lhcgr or PBS, Lhcgr from littermates + / + or Lhcgr + / - as comparison.
[0045] The results of immunofluorescence staining showed that Lhcgr in the AAV8-Lhcgr treatment group - / - There is a strong expression of Lhcgr in the interstitium of mouse testis, while Lhcgr injected in PBS - / - There was no expression of Lhcgr in mouse testis interstitium ( image 3 ).
[0046] Furthermore, compared with the PBS-injected group, Lhcgr treated with AAV8-Lhcgr - / - Serum testosterone levels were also significantly increased in mice. Its serum testosterone level can reach Lhcgr + / + or Lhcgr + / - Mou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com