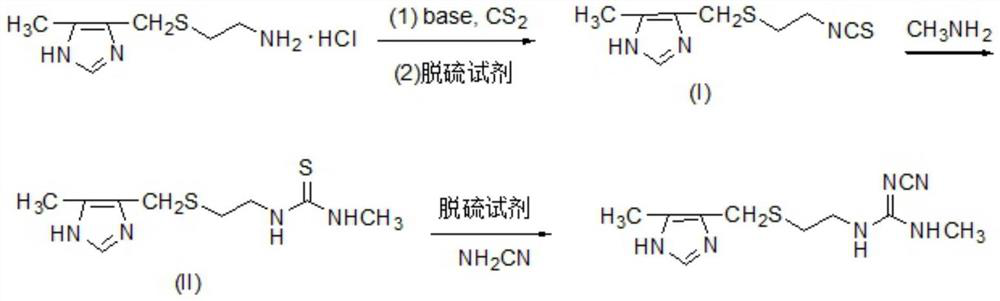
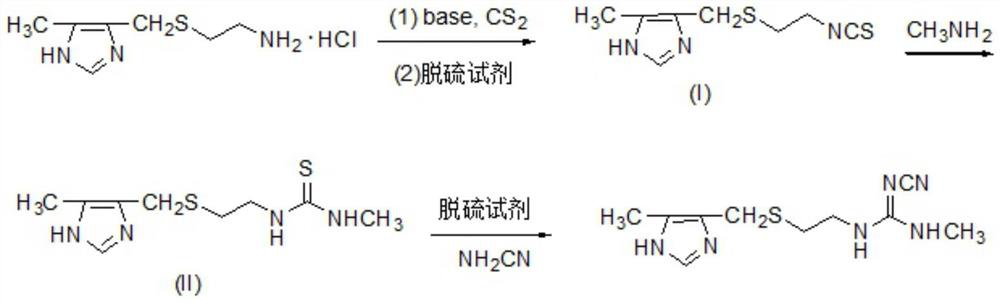
Preparation method of cimetidine
A technology of cimetidine and methylimidazole is applied in the field of preparation of cimetidine, can solve the problems of low production yield of cimetidine, existence of environmental pollution, a large amount of methyl mercaptan and the like, and achieves easy operation, The effect of low environmental pollution and short synthesis route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] 207.7 g (1 mol) 2- (5-methylimidazole-4-yl) methyl sulfide hydrochloride and 303 g (3.3 mol) Et3n were added to 2.5 L of ethyl acetate and water (volume ratio 3: 1) In the mixed solvent, stirring, the reaction liquid was cooled to 10-15 ° C, and 79.8 g (1.05 mol) CS2 was added dropwise, and the dropwise addition was added, the reaction was 1 h, and the temperature was 10 ° C, and 228 g (1.2 mol) pyrazoloxyl chloride, stir The reaction was 0.5 h and then at room temperature for 4 h. The filtrate was transferred to pH 2-3 with 6N hydrochloride, and the organic layer was separated, the aqueous layer was extracted with ethyl acetate, dried over anhydrous sodium sulfate, filtered, concentrated, decompression distillation, and intermediate (I). The aqueous phase was alkated with 30% NaOH solution, and the ET3N was recovered by distillation. The intermediate (I) yield was 94.3%, and the ET3N recovery was 79.3%. 1HNMR (400 MHz, CDCl3) δ2.35 (S, 3H), 2.54-2.61 (m, 2H), 3.70 (S, 2H),...
Embodiment 2
[0020] 207.7 g (1 mol) 2- (5-methylimidazole-4-yl) methyl sulfide hydrochloride and 496.8 g (3.6 mol) K2CO3 were added to 3 l Chloromethane and water (volume ratio 3: 1) In the mixed solvent, stirring, the reaction liquid was cooled to 10-15 ° C, add 91.2 g (1.2 mol) CS2, add 0.5 h, and 50 ° C, 10 ° C, add 152 g (1.2 mol) chloride. The solution was stirred at 0.5 h, and then at room temperature for 6 h. The reaction solution was filtered, and the organic layer was separated, the aqueous phase was extracted with dichloromethane, dried over anhydrous sulfate, filtered, concentrated, decreased pressure distillation, the intermediate (I), yield 93.6%.
[0021] 213 g (1 mol) intermediate (I) to 2.5 L of mass fraction of 60% ethanol, stirred, a methylamine solution of 40% of mass fraction was added 116.2 g (1.5 mol), and reacted at 40-50 ° C for 2-3 hours. Concentrate under reduced pressure, cool the crystallization, and crude product. Ethanol recrystallization and produce intermediate ...
Embodiment 3
[0024] 207.7 g (1 mol) 2- (5-methylimidazole-4-yl) methyl sulfide hydrochloride and 360.4 g (3.4 mol) Na2CO3 were added to mixed solvents of toluene and water (volume ratio 3: 1). In the mixing, stirring, the reaction liquid was cooled to 10-15 ° C, and 83.6 g (1.1 mol) CS2 was added dropwise, and the dropwise addition was 0.5-1 h, and the temperature was 10 ° C, and 417.9 g (1.2 mol) of a water sulfate saturation. The solution was stirred for 0.5 h, and then at room temperature for 5 h. The reaction liquid was filtered, and the organic layer was separated, the aqueous phase was extracted with toluene, dried over anhydrous sodium sulfate, concentrated, decomposed distillation, the intermediate (I), yield 94.0%.
[0025] 213 g (1 mol) intermediate (I) was added to a 2.5 L mass fraction of 65% ethanol aqueous ethanol, stirred, and an methylamine solution of a mass fraction of 40% was added to 155 g (2.0 mol), and reacted at 40-50 ° C for 2-3 hours. Concentrate under reduced pressure...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com