Application of pterostilbene in preparation of medicine for preventing and/or treating intestinal epithelial barrier injury
A technology for barrier damage and pterostilbene, which is applied in the application field of pterostilbene in the preparation of drugs for preventing and/or treating intestinal epithelial barrier damage, can solve problems such as undocumented PTE, and achieve the effect of reducing epithelial integrity damage in vitro
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Protective effect of pterostilbene (PTE) on intestinal epithelial cells
[0047] 1. Culture of human colon cancer Caco2 cells
[0048] (1) Resuscitating cells: Take out the frozen Caco2 cell line from the liquid nitrogen tank, put it into warm water at 37°C and dissolve it quickly, then add the dissolved cell solution into a 15mL centrifuge tube containing 4mL of DMEM medium, and then Centrifuge at 1500rpm / min for 3min, discard the supernatant after the centrifugation is completed, add 1mL of DMEM medium and blow gently to suspend the cells in the medium, then add the cell suspension into a petri dish containing 5mL of DMEM medium, with the word "ten" Shake gently and place in 37°C containing 5% CO 2 Cultured in an incubator.
[0049] (2) Culture conditions: 37°C, 5% CO 2 , the culture medium is DMEM containing 10% fetal bovine serum (FBS), 2mg / L insulin and 1% penicillin and streptomycin mixed solution, and the culture medium needs to be changed every day.
[0050]...
Embodiment 2
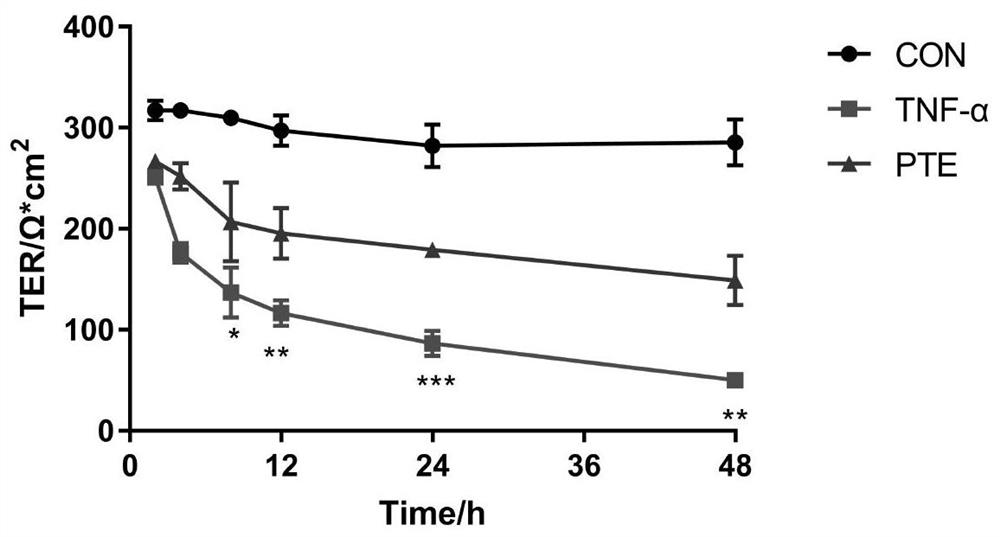
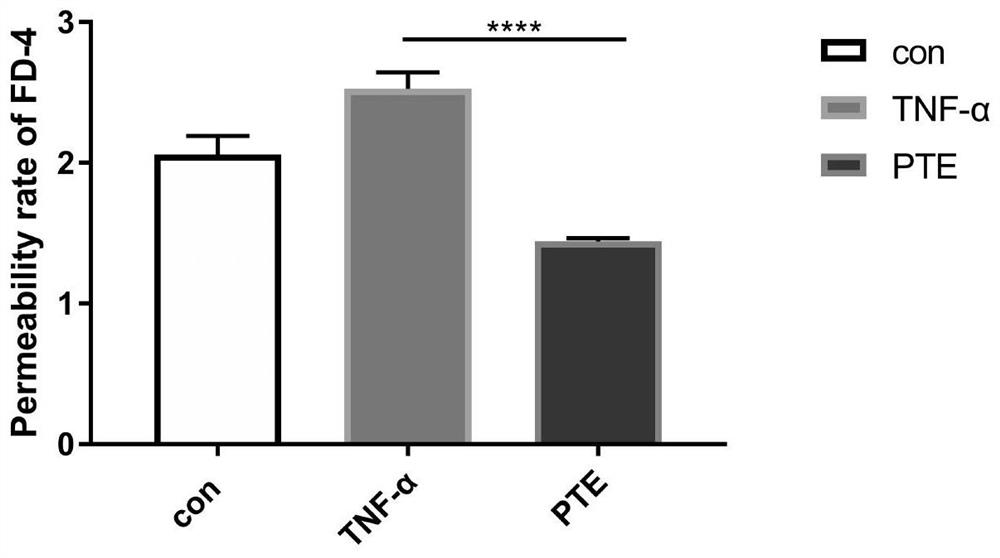
[0064] cell monolayer permeability
[0065] The cell monolayer permeability is another important factor to measure whether the in vitro barrier model is damaged. It measures the passage rate of the fluorescently labeled macromolecular substance FITC-dextran (FITC-dextran) through the monolayer cells, which is used to evaluate the monolayer cell permeability.
[0066] The tight connection model established in Example 1 is used. After Caco2 cells were differentiated for 21 days, the Transwell chamber was slowly washed twice with PBS solution pre-warmed at 37°C, and then 100 μL of 0.1 mg / mL FITC was added to the AP layer of the Transwell chamber, and 600 μL of PBS buffer was added to the BL layer. in CO 2 After incubating in the incubator for 1 h, take 100 μL of liquid on the BL side and AP side of the Transwell respectively, and calculate the fluorescence absorbance with a multi-functional microplate reader (where the excitation wavelength is set at 480 nm, and the emission wa...
Embodiment 3
[0077] Study on the protective effect of PTE on the intestinal epithelial barrier in mice
[0078] Health Evaluation of a Mouse Model of Intestinal Injury
[0079] Experimental animal culture
[0080] 8-10 weeks old male C57BL / 6 mice, weighing 20-23 g, were purchased from Beijing Huafukang Biotechnology Co., Ltd. All the mice were placed in the Animal Experiment Center of the Institute of Radiation Medicine, Chinese Academy of Medical Sciences, raised at a room temperature of 23±3°C and a humidity of 50%±10%. Any disease was used in this experiment. All animal experiments were approved by the Ethics Committee of Tianjin University of Commerce.
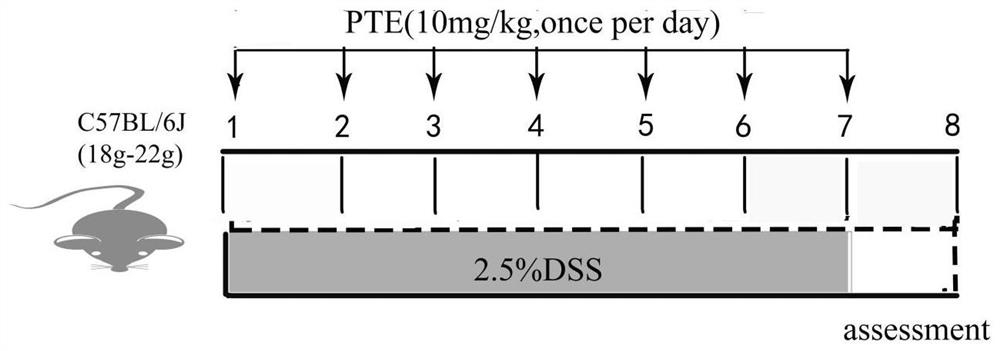
[0081] Establishment of mouse intestinal injury model
[0082] Mice were randomly divided into 3 groups: normal control group, dextran sulfate sodium salt (DSS, molecular weight: 36-50kDa) treatment group, PTE group, 10 mice in each group. The blank control group had a normal diet, and the other two groups added 2.5% DSS to the drin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com