Amphiphilic oligopeptide structure substance
An amphiphilic and oligopeptide technology, applied in the field of biomedicine, can solve the problem of not being able to obtain compounds or polymers, and achieve the effect of improving biological activity and increasing intake
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1, synthesis (LLHH) n oligopeptide:
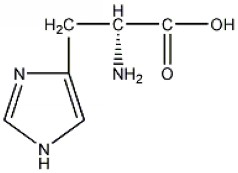
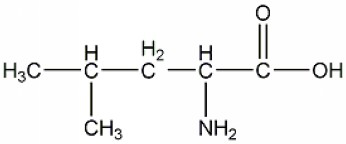
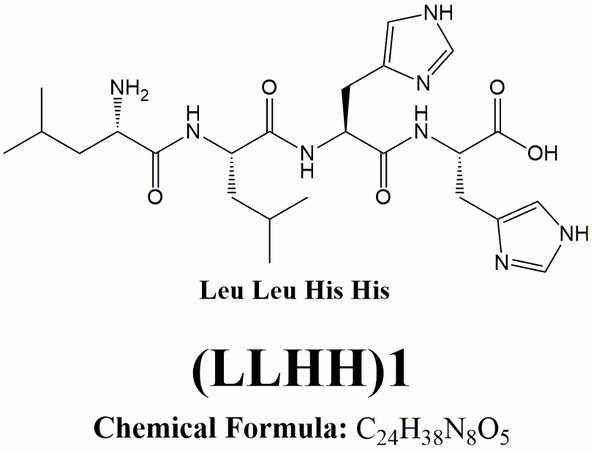
[0048] In the present invention, according to the structural characteristics of the cell inclusion body and the understanding of the mechanism of drug escape from the inclusion body, a kind of leucine-leucine-histidine was obtained by designing and screening by Fmoc polypeptide solid-phase synthesis technology -The basic sequence of histidine (LLHH) is the amphipathic oligopeptide structure of the mother nucleus sequence, in which the molecular structural formula of leucine (Leucine, Leu, L) is figure 1 ; The molecular structural formula of histidine (Histidine, His, H) is figure 2 ; Leucine-leucine-histidine-histidine (LLHH) basic sequence composition of the mother core sequence is image 3 ;Based on the mother nucleus sequence, the polymer leucine-leucine-histidine-histidine oligopeptide structure ((LLHH)n, where n is 1, 2, 3, 4, 5, 6, 7, 8 or 9; the structure of the 3-mer leucine-leucine-histidine-histidine ((LLHH)3...
Embodiment 2
[0049] Example 2. Construction of a tumor-targeted nucleic acid delivery system cRGD-R9-(LLHH)n / siRNA:
[0050] Integrin αvβ3 receptors are only expressed on tumor neovascular endothelial cell membranes and some tumor cell membranes, and are commonly used delivery targets for the study of small nucleic acid (siRNA) and other drugs for targeted therapy of tumors; oligopeptide cRGD is a specific ligand for αvβ3 receptors Studies have found that cRGD can target drug molecules such as small nucleic acid (siRNA) carried by non-chemical bond encapsulation or chemical bond coupling into tumor cells expressing αvβ3 receptors, so as to achieve the purpose of drug killing tumor cells (Park J, Singha K, Son S, Kim J, Namgung R, Yun C O, Kim W J. A review of RGD-functionalized nonviral gene delivery vectors for cancer therapy[J]. CancerGene Ther,2012,19(11):741-748. ).
[0051] In order to increase the number of drugs escaping into the cytoplasm in the cRGD-mediated drug delivery system ...
Embodiment 3
[0052] Embodiment 3, obtain siRNA sequence:
[0053] The siRNA EGFR sequence (siEGFR) was designed and obtained by using bioinformatics technology, and the glioblastoma cell line U87MG was used as a model cell, and the expression of the candidate siRNA sequence to silence the target gene EGFR mRNA was detected by RT-qPCR method, and the best activity was obtained. siEGFR sequence. Liposome transfection of 100 nM siEGFR, after 48 hours, the efficiency of silencing EGFR mRNA in U87 cells was as high as more than 85%, and was used as a model molecule of the present invention for testing cRGD-R9-(LLHH containing (LLHH)n structure ) n / siRNA gene silencing efficiency.
[0054] Negative control siNC sense strand sequence (5'-3') is: CGUGAUUGCGAGACUCUGAdTdT;
[0055] The sequence of the antisense strand (5'-3') is: UCAGAGUCUCGCAAUCACGdTdT.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com