A kind of bovine serum albumin self-assembled triphenylamine photosensitizer and preparation method and application
A technology of bovine serum albumin and triphenylamine, applied in carboxylate preparation, photodynamic therapy, carboxylate preparation, etc., can solve problems such as side effects and loss of normal organ function, and achieve good biocompatibility and low dark toxicity , the effect of obvious application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
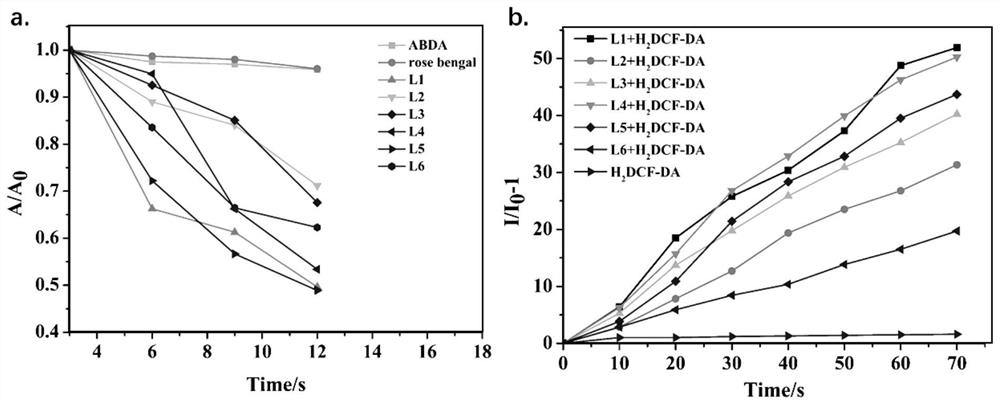
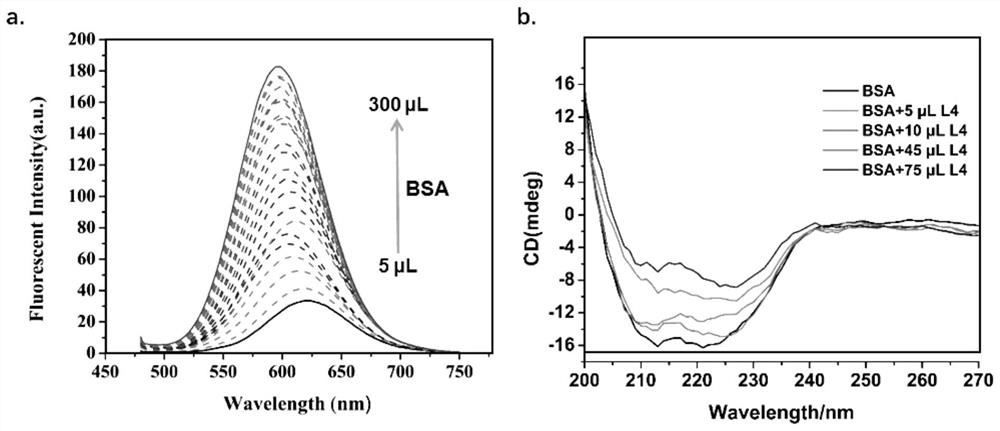
[0066] A bovine serum albumin self-assembled triphenylamine-based photosensitizer in this embodiment is formed by self-assembly of an organic small molecule photosensitizer L and BSA; the organic small molecule photosensitizer L is a triphenylamine derivative containing a bipyridine salt, It forms BSA@L nanoparticles by self-assembly with BSA.
[0067] In this embodiment, the triphenylamine derivatives containing bipyridine salts are specifically 4-bis[(N,N-diphenylamine)styryl]-N-[1,2-bis(ethoxy)ethyl yl]-4-dipyridine iodide salt, 4-bis[(N,N-diphenylamine)styryl]-N-[1,2-bis(ethoxy)ethyl]-4-dipyridine Nitrate, 4-bis[(N,N-diphenylamine)styryl]-N-[1,2-bis(ethoxy)ethyl]-4-dipyridine-4-methylbenzenesulfonic acid acid salt, 4-bis[(N,N-diphenylamine)styryl]-N-[1,2-bis(ethoxy)ethyl]-4-dipyridine hexafluorophosphate, 4- Bis[(N,N-diphenylamine)styryl]-N-[1,2-bis(ethoxy)ethyl]-4-dipyridine trifluoroacetate, 4-bis[(N ,N-diphenylamine) styryl]-N-[1,2-bis(ethoxy)ethyl]-4-dipyridine tetr...
Embodiment 2
[0072] A preparation method of the BSA@L1 nanophotosensitizer in the above-mentioned embodiment 1 of the present embodiment comprises the following steps:
[0073] (1) Preparation of intermediate M1
[0074] Weigh 1,2-bis(2'-iodoethoxy)-ethane (1.01 g, 2.7 mmol) and 4-picoline (0.76 g, 8.1 mmol), dissolve in 20.0 mL of absolute ethanol, 60 Reflux at ~80°C, and the reaction was traced by thin layer chromatography (TLC); after 24 hours of reaction, the reaction was completed, and column chromatography was performed to obtain a white solid M1, namely 4-dimethyl-N-[1,2-bis( Ethoxy)ethyl]-4-dipyridinium iodide salt.
[0075] Yield: 64.7%. 1 H NMR (400MHz, DMSO-d 6 ),δ(ppm):8.88-8.86(d,J=8.00Hz,4H),8.02-8.01(d,J=4.00Hz,4H),4.73-4.70(t,J=6.00Hz,4H),3.85 -3.83(t, J=4.00Hz, 4H), 3.49(s, 4H), 2.64(s, 6H);13 C NMR (100MHz, DMSO-d 6 ), δ(ppm): 159.12, 144.06, 128.03, 69.42, 68.62, 59.45, 21.47; MS(ESI): calcd for: C 18 H 26 N 2 O 2 2+ [M / 2], 151.10; found, 151.0982.
[0076] (2...
Embodiment 3
[0083] A preparation method of the BSA@L2 nanophotosensitizer in the above-mentioned embodiment 1 of the present embodiment comprises the following steps:
[0084] (1) Preparation of intermediate M1
[0085] Same as step (1) of Example 2.
[0086] (2) Preparation of L1
[0087] With the step (2) of embodiment 2.
[0088] (3) Preparation of L2
[0089] Silver nitrate (0.064g, 0.38mmol) was dissolved in 20mL of anhydrous acetonitrile solution, then L1 (0.20g, 0.19mmol) was added to the reaction system, refluxed at 60-80°C for 4h; after the reaction was over, the precipitate was removed by filtration, and the filtrate was concentrated , to obtain 0.12g of red product L2, namely 4-bis[(N,N-diphenylamine)styryl]-N-[1,2-bis(ethoxy)ethyl]-4-dipyridine nitric acid Salt.
[0090] Yield: 66.7%. 1 H NMR (400MHz, DMSO-d 6 ),δ(ppm):8.72-8.71(d,J=4.00Hz,4H),8.08-8.07(d,J=4.00Hz,4H),7.87-7.83(d,J=16.00Hz,2H),7.55 -7.53(d,J=8.00Hz,4H),7.34-7.31(t,J=8.00Hz,8H),7.22-7.18(d,J=16.00Hz,2H)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com