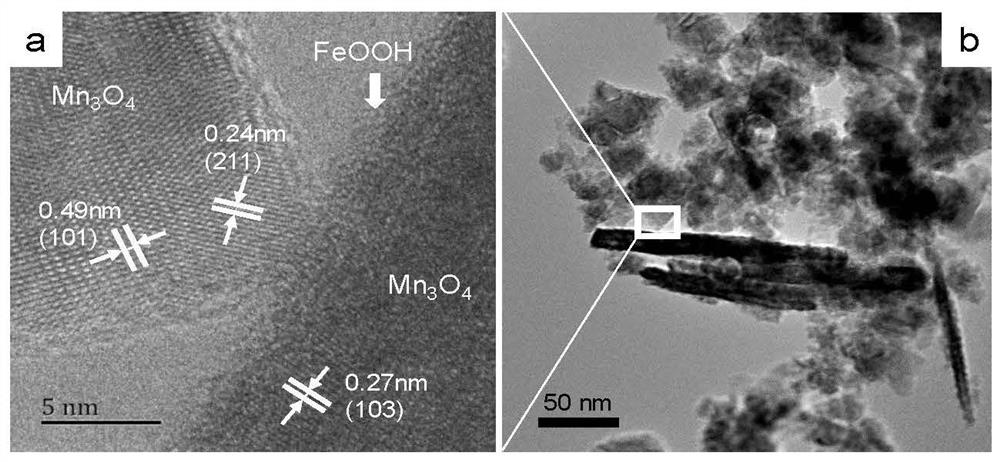
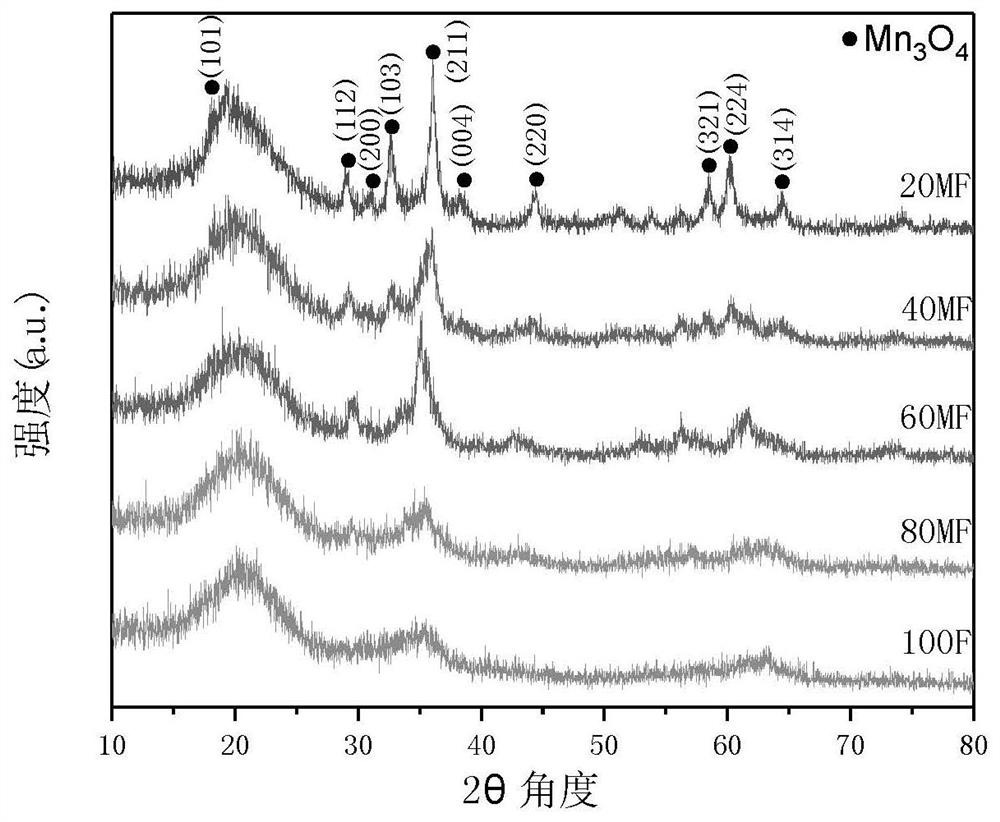
FeOOH coated Mn3O4 composite material as well as preparation method and application thereof
A composite material and reaction technology, applied in the direction of catalyst activation/preparation, chemical instruments and methods, water/sludge/sewage treatment, etc., can solve the problems of low oxidant utilization efficiency and slow reaction rate, etc., and achieve sustainable recycling Use, fast response rate, obvious effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Fe
[0041] Preparation example FeOOH coated Mn 3 o 4 Preparation of composite materials
[0042] Include the following steps: (1) with 18mmol NaOH and a certain amount of K 2 FeO 4 Dissolve in 30mL deionized water in turn to form A liquid; (2) 9mmol MnCl 2 4H 2 O was dissolved in 70mL of deionized water, continuously stirred and heated in a water bath to 90°C to form liquid B; (3) Pour the prepared liquid A into liquid B drop by drop, and after stirring and reacting at 90°C for 2 hours, the prepared complex The solution was cooled to room temperature, and the unreacted ions in the solution were washed away with deionized water and absolute ethanol, and dried at 70°C to constant weight, that is, FeOOH-coated Mn 3 o 4 composite material.
[0043] In order to investigate different K 2 FeO 4 The effect of dosage on the final product activated PMS to remove dye, while keeping MnCl 2 4H2 Under the premise of constant O dosage, according to the mass ratio of theoretical FeOOH t...
Embodiment 1
[0044] Embodiment 1 Separate PMS degrades RhB
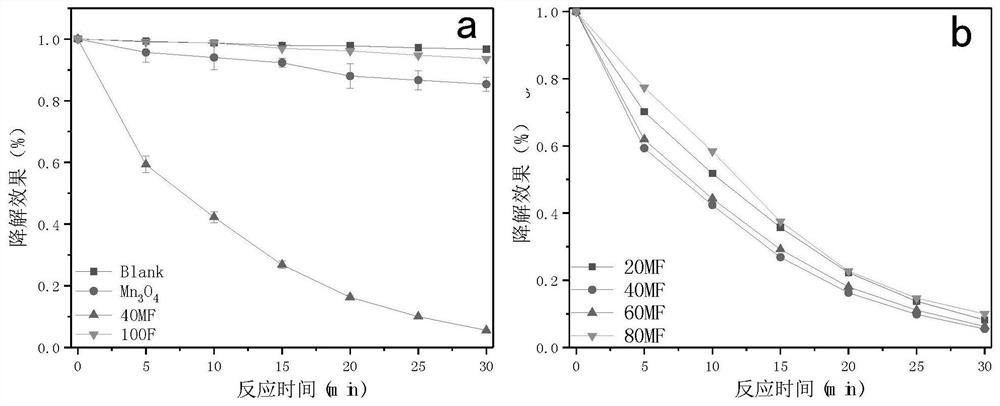
[0045] Pipette 5mL of the RhB stock solution (1g / L) into a 250mL beaker, put it into the rotor and stir in a constant temperature water bath for 30min. Then take 1mL of PMS stock solution (20g / L) and add it dropwise to the beaker, use boric acid (0.2M) and sodium tetraborate (0.1M) to adjust the pH value of the solution to 4, and replenish the volume of the adjusted solution to 100mL. At intervals of 5 minutes, take 2 mL of the solution with a syringe, quench the unreacted PMS with sodium thiosulfate after filtering with a 0.45 μm filter membrane, and detect the absorbance of the remaining pollutants, draw the pollutant degradation curve, and the results are as follows image 3 as shown in a.
Embodiment 2
[0046] Example 2 FeOOH coated Mn 3 o 4 (0:1) Composites activate PMS to degrade RhB
[0047] Weigh 20mg FeOOH coated Mn 3 o 4 (0:1) The composite material was sonicated in 80mL water for 5min, and after being uniformly dispersed, it was added to a 250mL beaker together with 5mL of RhB stock solution (1g / L), placed in the rotor and stirred in a constant temperature water bath for 30min. Then take 1mL of PMS stock solution (20g / L) and add it dropwise to the beaker, use boric acid (0.2M) and sodium tetraborate (0.1M) to adjust the pH value of the solution to 4, and replenish the volume of the adjusted solution to 100mL. At intervals of 5 minutes, take 2 mL of the solution with a syringe, quench the unreacted PMS with sodium thiosulfate after filtering with a 0.45 μm filter membrane, and detect the absorbance of the remaining pollutants, draw the pollutant degradation curve, and the results are as follows image 3 as shown in a.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com