Iron-based compound as well as preparation method and application thereof
A compound, iron-based technology, applied in the direction of iron-organic compounds, refining with oxygen-containing compounds, chemical instruments and methods, etc., can solve problems such as poor results, and achieve short reaction time and good repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0026] This specific embodiment also proposes a preparation method of an iron-based compound, comprising the following steps:
[0027] Mix 2,6 diisopropylaniline, 40%-45% glyoxal, formic acid and a third organic solvent, then react 1-2d, filter, and wash with cold methanol to obtain the diazepine; Wherein, the molar ratio of the 2,6-diisopropylaniline to the glyoxal is 2-3:1; the third organic solvent is anhydrous methanol;
[0028] Stir paraformaldehyde and HCl (4M in dioxane) at 30-60°C for 4-12h, then add a mixture of diazebutadiene and THF, and continue to stir the reaction at room temperature for 4-5h, filter and wash to obtain the 1,3-bis(2,6-diisopropyl-1-phenyl) imidazolium chloride; the molar ratio of the diazebutadiene, the paraformaldehyde and the HCl is 1:( 1-1.5): (1-1.5); It should be noted that the HCl is obtained by dissolving hydrogen chloride gas in dioxane;
[0029] Mix 1,3-bis(2,6-diisopropyl-1-phenyl)imidazolium chloride, potassium tert-butoxide and the ...
Embodiment 1
[0040] The iron-based compound proposed in this embodiment is prepared by the following steps:
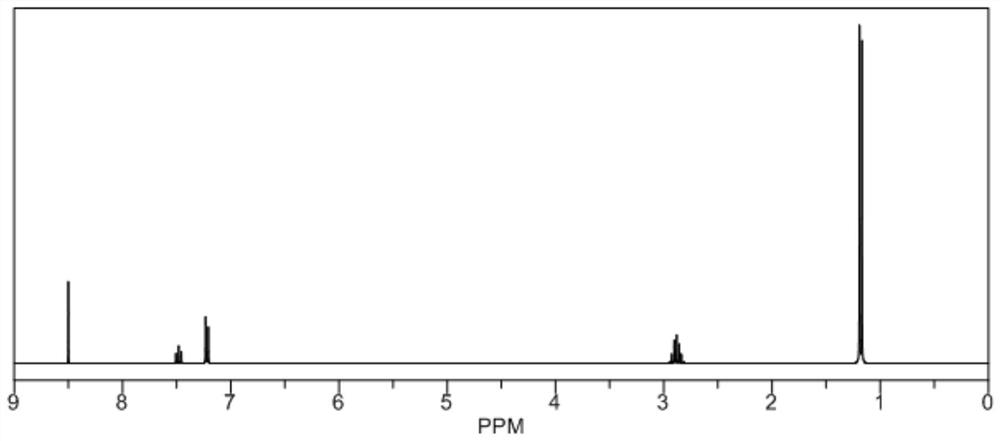
[0041] Add 2,6 diisopropylaniline, 40% glyoxal, and formic acid into the third organic solvent absolute ethanol to react for 2d, filter, and wash with cold methanol to obtain the diazebutadiene; wherein, the The molar ratio of 2,6-diisopropylaniline to the glyoxal is 2:1; the yield of diazepine is 89.2%; figure 1 In, diazebutadiene 1 H-NMR (400MHz, CDCl 3 ): δl1.28(d, J=7.6Hz, 24H, CH(CH 3 ) 2 ,3.03(sep,J=6.4Hz,4H,CH(CH 3 ) 2 ),7.27(m,6H,(CH(CH 3 ) 2 ) 2 -C 6 h 3 ),8.19(s,2H,NCH), figure 1 Illustrates that diazebutadiene is obtained;
[0042] Stir paraformaldehyde and HCl (4M in dioxane) at 30°C for 12h, then add diazebutadiene and THF to the mixture, and continue to stir the reaction at room temperature for 4h, and filter and wash to obtain the 1, 3-bis(2,6-diisopropyl-1-phenyl)imidazolium chloride; the molar ratio of the diazebutadiene, the paraformaldehyde and the H...
Embodiment 2
[0046] The iron-based compound of the present embodiment is prepared by the following steps:
[0047] Add 2,6 diisopropylaniline, 45% glyoxal, and formic acid into the third organic solvent absolute ethanol to react for 1.5 days, filter and wash with cold methanol to obtain the diazebutadiene; wherein, the The molar ratio of said 2,6-diisopropylaniline to said glyoxal is 2.5:1; the productive rate of diazepine is 88.7%;
[0048] Stir paraformaldehyde and HCl (4M in dioxane) at 40°C for 12h, add it to the mixture of diazetidine and THF, then continue to stir the reaction at room temperature for 8h, and obtain the 1,3- Bis(2,6-diisopropyl-1-phenyl)imidazolium chloride; the molar ratio of said diazebutadiene, said paraformaldehyde and said HCl is 1:1.5:1; 1 , The yield of 3-bis(2,6-diisopropyl-1-phenyl)imidazolium chloride was 87.2%;
[0049] Mix 1,3-bis(2,6-diisopropyl-1-phenyl)imidazolium chloride and potassium tert-butoxide at a molar ratio of 2:1 and add to the first organi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com