A kind of triptolide self-dissolving microneedle
A technology of triptolide and self-dissolving microneedles, which is applied in the directions of microneedles, needles, organic active ingredients, etc., achieves the effects of good formability, stable and feasible process, and good mechanical strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Example 1, a triptolide self-dissolving microneedle, according to parts by weight, the tip matrix prescription of the triptolide self-dissolving microneedle is 24 parts of PVP, 7.8 parts of PVA, 0.45 parts of CMC-Na, 0.28 parts of triptolide, 40 parts of distilled water and 20 parts of absolute ethanol; the prescription of the backing layer matrix of the triptolide self-dissolving microneedle is 32 parts of PVP, 9 parts of PVA and 53 parts of distilled water.
[0067] Triptolide self-dissolving microneedles are prepared according to the following steps:
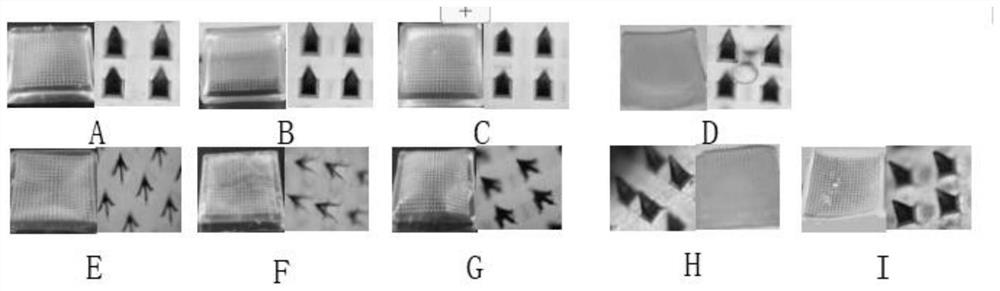
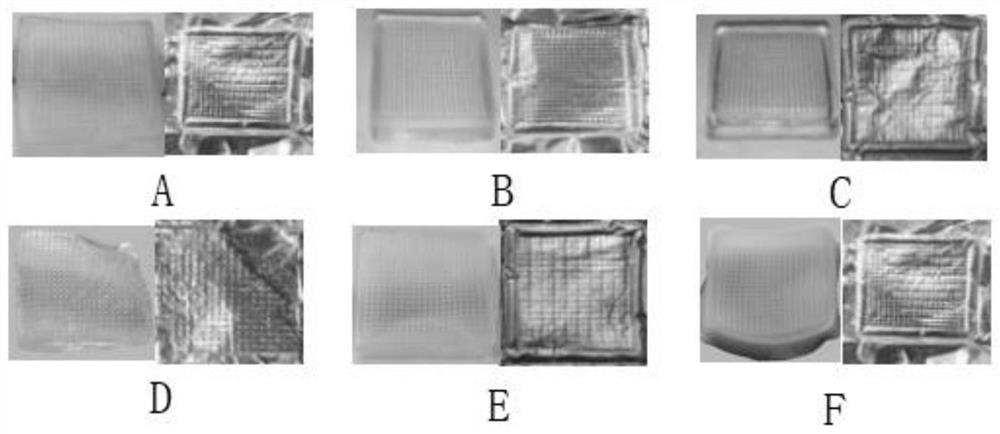
[0068] (1) Preparation of needles: According to the needle tip matrix prescription of triptolide self-dissolving microneedles, the needle tip materials were weighed and fully dissolved and stirred evenly, then poured into the microneedle mold, centrifuged at 3900r / min for 8min, removed and collected on the surface of the microneedle mold The drug-containing matrix solution was put into a desiccator to dry for 5 hours t...
Embodiment 2
[0070] Example 2, a triptolide self-dissolving microneedle, in parts by weight, the tip matrix prescription of the triptolide self-dissolving microneedle is 29 parts of PVP, 8.3 parts of PVA, 0.65 parts of CMC-Na, 0.42 parts of triptolide, 45 parts of distilled water and 23 parts of absolute ethanol; the prescription of the backing layer matrix of the triptolide self-dissolving microneedle is 37 parts of PVP, 12 parts of PVA and 58 parts of distilled water.
[0071] Triptolide self-dissolving microneedles are prepared according to the following steps:
[0072] (1) Preparation of needles: according to the needle tip matrix prescription of triptolide self-dissolving microneedles, the needle tip materials were weighed and fully dissolved, stirred evenly, poured into the microneedle mold, centrifuged at 4100r / min for 12min, and removed and collected on the surface of the microneedle mold. The drug-containing matrix solution was put into a desiccator to dry for 7 hours to obtain a ...
Embodiment 3
[0074] Example 3, in parts by weight, the tip matrix prescription of the triptolide self-dissolving microneedle is 26 parts of PVP, 8 parts of PVA, 0.5 part of CMC-Na, 0.3 part of triptolide, 42 parts of distilled water and no 21 parts of water ethanol; the prescription of the backing layer matrix of the triptolide self-dissolving microneedle is 34 parts of PVP, 10 parts of PVA and 55 parts of distilled water.
[0075] Triptolide self-dissolving microneedles are prepared according to the following steps:
[0076] (1) Preparation of needles: according to the needle tip matrix prescription of triptolide self-dissolving microneedles, weigh the needle tip material to fully dissolve and stir evenly, inject it into the microneedle mold, centrifuge at 3950r / min for 9min, remove and collect the microneedle mold surface The drug-containing matrix solution was put into a desiccator to dry for 5.5 hours to obtain a microneedle mold with drug-containing needle tip;
[0077] (2) Preparati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com