Method for realizing quantification of D-dimer in plasma based on immobilized metal ion affinity chromatography enrichment
A technology for immobilizing metals and dimers, which is applied in the direction of measuring devices, material separation, material inspection products, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
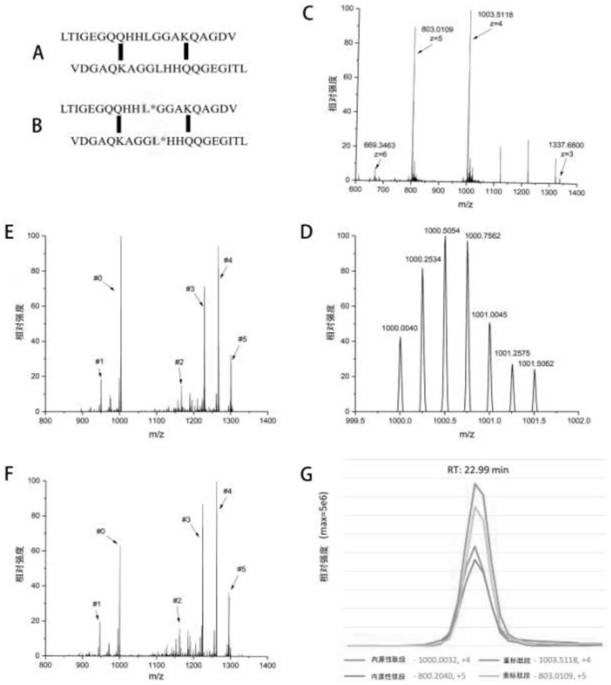
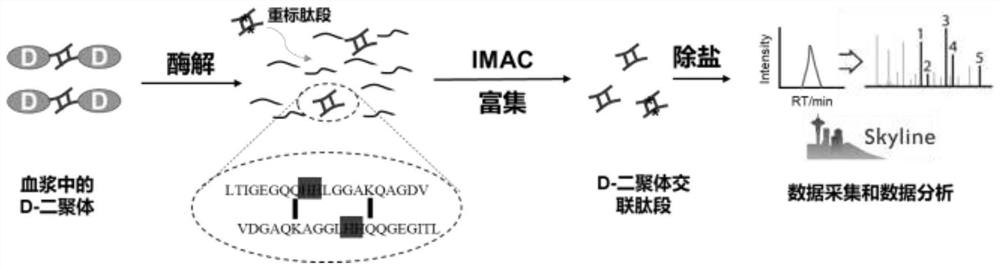
[0035] Enrichment of D-dimer in plasma by immobilized metal ion affinity chromatography and quantitative detection by mass spectrometry:
[0036] (1) Take 8 μL of plasma sample containing D-dimer, dissolve 200 μL in 50 mM Hepes (4-hydroxyethylpiperazineethanesulfonic acid) buffer solution (pH 8.0) containing 8M urea, and add a final concentration of 20 mM DTT (dithiothreitol), in a water bath at 60°C for 1h, then add IAA (iodoacetamide) at a final concentration of 40mM, and react in the dark at 25°C for 40min;
[0037] (2) Transfer the sample obtained after the treatment in step (1) to the ultrafiltration membrane of an ultrafiltration tube with a molecular weight cut-off of 3K, centrifuge at 14000g for 30min to remove the denaturing solution, and then wash twice with 400μL of 50mM Hepes buffer, Centrifuge at 14,000 g for 30 min to remove, add 200 μL of 50 mM Hepes buffer, add trypsin with an enzyme:protein mass ratio of 1 / 20, enzymolyze in a water bath at 37°C for 12 hours, a...
Embodiment 2
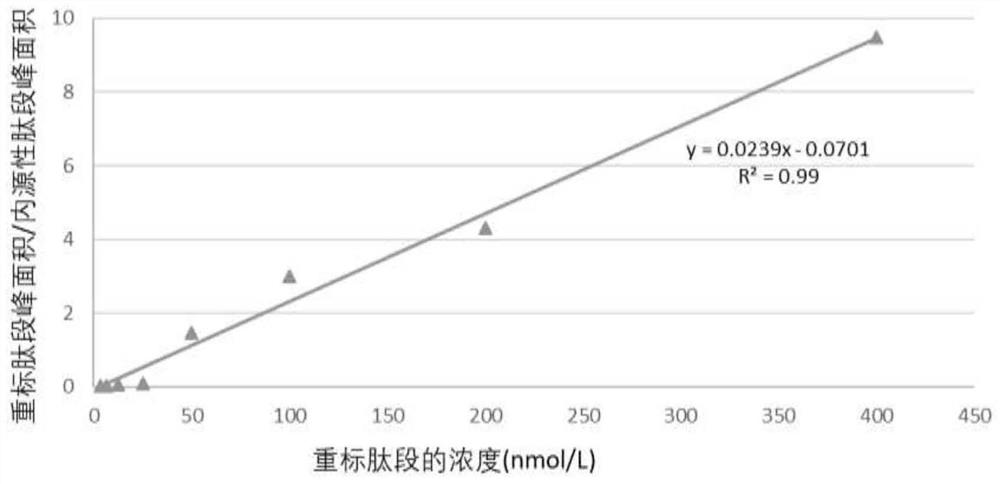
[0049] Calibration curve of D-dimer in plasma enriched by immobilized metal ion affinity chromatography and quantitatively detected by mass spectrometry:
[0050] The operation procedure is the same as that in Example 1, except that 33 identical samples are processed, and 0, 5, 10, 25, 50, 100, 200, 400, 800, 1600, and 3200 fmol are added at the place where the heavy-labeled peptide is added in step (5), and each concentration has three repetitions. During data processing, the extracted chromatographic peak area of the heavy-labeled peptide was divided by the chromatographic peak area of the endogenous cross-linked peptide to obtain the relative amount of the heavy-labeled cross-linked peptide in the sample, and a calibration curve was drawn.
[0051] image 3 A calibration curve for the described IMAC enrichment method combined with PRM mass spectrometry detection mode for D-dimer protein in plasma. By adding different concentrations of heavy-labeled cross-linked peptide...
Embodiment 3
[0053] Using immobilized metal ion affinity chromatography to enrich D-dimer in plasma and to investigate the reproducibility of mass spectrometry quantitative detection method:
[0054] The same plasma sample was prepared four times a day according to the operating procedure in Example 1 for three days for detection and quantification. During data processing, the extracted chromatographic peak area of the relabeled peptide was divided by the chromatographic peak area of the endogenous crosslinked peptide to obtain the relative amount of the relabeled crosslinked peptide in the sample, and then the inter-batch and intra-batch reproducibility was calculated.
[0055] The inter-assay and intraassay reproducibility of the IMAC enrichment method described in Table 1 combined with the PRM mass spectrometry detection mode for the quantification of D-dimer in plasma. From the CV value of the quantitative results (standard≤15%), it can be seen that the quantitative method has good...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com