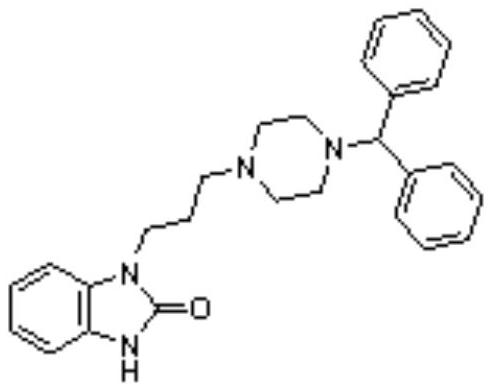
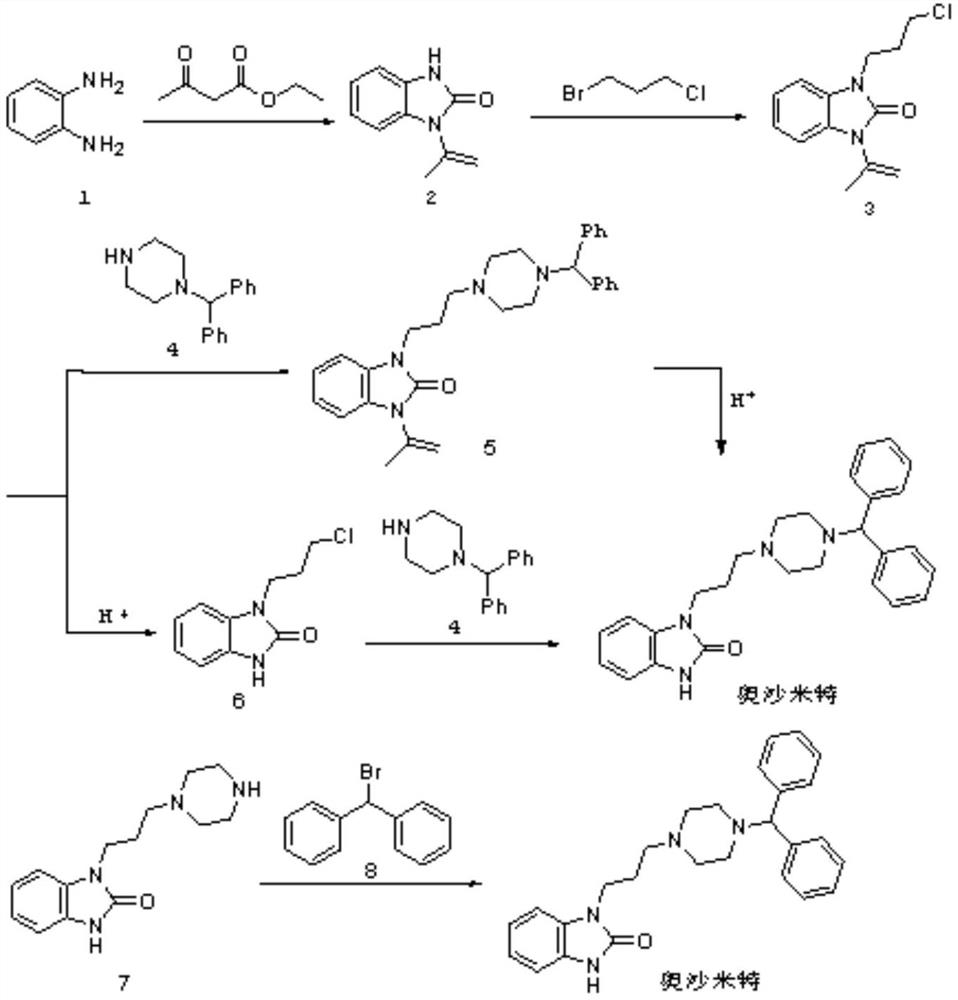
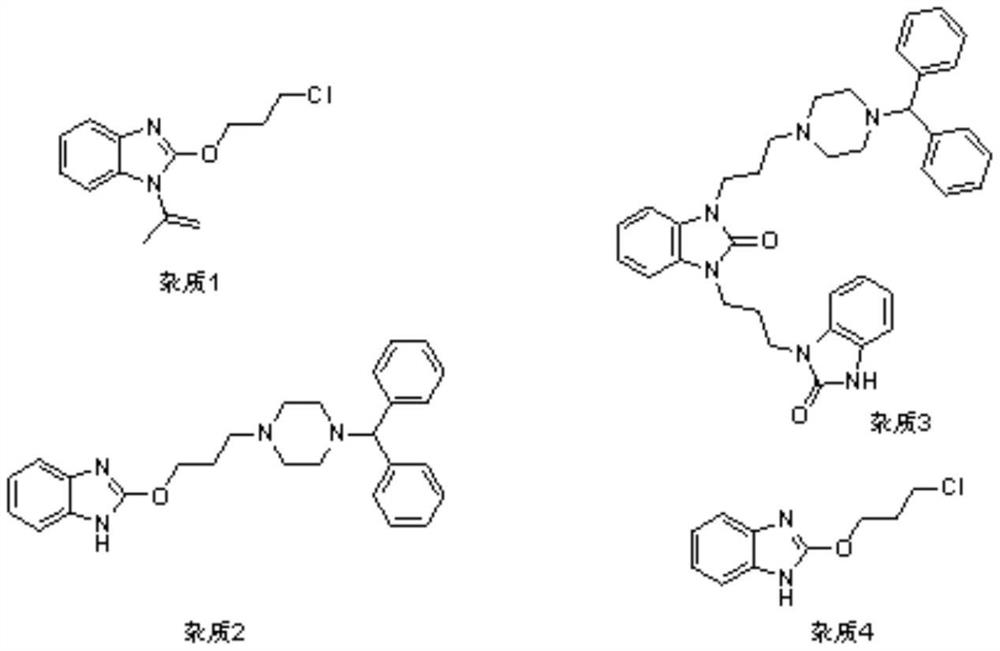
Synthetic method of oxatomide
A synthetic method and ethoxylate technology, applied in the direction of organic chemistry, etc., can solve the problems of harshness, difficult operation, harsh reaction conditions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Add 2-ethoxy-1H-benzimidazole, 1-(3-chloropropyl)-4-benzhydrylpiperazine dihydrochloride, sodium iodide and acetone successively in 50L reactor, then in Add potassium carbonate, 2-ethoxy-1H-benzimidazole, 1-(3-chloropropyl)-4-benzhydrylpiperazine dihydrochloride, sodium iodide and inorganic base slowly under stirring The molar ratio is 1:1.2:0.1:3.3. After the addition is completed, the temperature is raised to 55-60°C for reaction, and the reaction is refluxed for 10 hours; after the detection reaction is completed, the acetone is recovered by concentration under reduced pressure, cooled, and 20L of water is added to the residue, and stirred at room temperature for 2 Hour, suction filtration, washing with water, drying, obtain white solid product 1-[3-(4-benzhydryl-1-piperazinyl) propyl group]-2-ethoxy-1H-benzimidazole, yield 74.9%.
[0031] Dissolve 1-[3-(4-benzhydryl-1-piperazinyl)propyl]-2-ethoxy-1H-benzimidazole in isopropanol, add concentrated hydrochloric acid, ...
Embodiment 2
[0034]Add 2-ethoxy-1H-benzimidazole, 1-(3-chloropropyl)-4-benzhydrylpiperazine dihydrochloride, sodium iodide and isopropanol successively in the 50L reactor, Then slowly add sodium carbonate, 2-ethoxy-1H-benzimidazole, 1-(3-chloropropyl)-4-benzhydrylpiperazine dihydrochloride, sodium iodide and inorganic The molar ratio of the base is 1:1.0:0.03:3.8. After the addition, the temperature is raised to 55-60°C for reaction, and the reaction is refluxed for 10 hours; after the detection reaction is completed, the isopropanol is recovered by concentration under reduced pressure, cooled, and 20L of water is added to the residue , stirred at room temperature for 2 hours, suction filtered, washed with water, and dried to obtain a white solid product 1-[3-(4-benzhydryl-1-piperazinyl)propyl]-2-ethoxy-1H-benzo Imidazole, yield 67.1%.
[0035] Dissolve 1-[3-(4-benzhydryl-1-piperazinyl)propyl]-2-ethoxy-1H-benzimidazole in ethanol, add concentrated hydrochloric acid, 1-[3-( The molar rati...
Embodiment 3
[0038] Add 2-ethoxy-1H-benzimidazole, 1-(3-chloropropyl)-4-benzhydrylpiperazine dihydrochloride, sodium iodide and tetrahydrofuran successively in 50L reactor, then in Slowly add sodium hydroxide, 2-ethoxy-1H-benzimidazole, 1-(3-chloropropyl)-4-benzhydrylpiperazine dihydrochloride, sodium iodide and inorganic base under stirring The molar ratio is 1:1.5:0.01:4.0. After the addition, the temperature is raised to 55-60°C for reaction, and the reaction is refluxed for 10 hours; after the detection reaction is completed, the tetrahydrofuran is recovered by concentration under reduced pressure, cooled, and 20L of water is added to the residue, and stirred at room temperature After 2 hours, filter with suction, wash with water, and dry to obtain the white solid product 1-[3-(4-benzhydryl-1-piperazinyl)propyl]-2-ethoxyl-1H-benzimidazole. The rate is 76.7%.
[0039] Dissolve 1-[3-(4-benzhydryl-1-piperazinyl)propyl]-2-ethoxy-1H-benzimidazole in tert-butanol, add concentrated hydrochlo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com