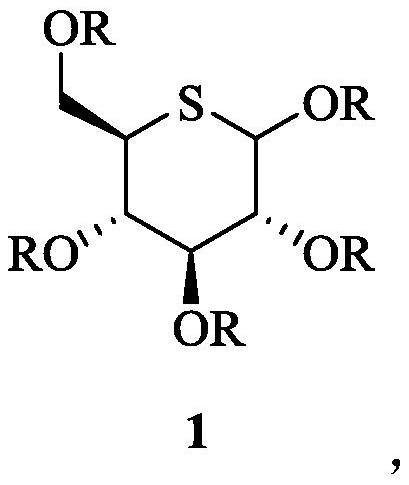
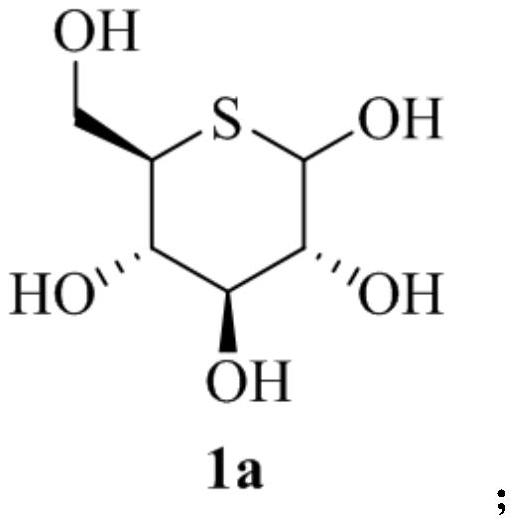
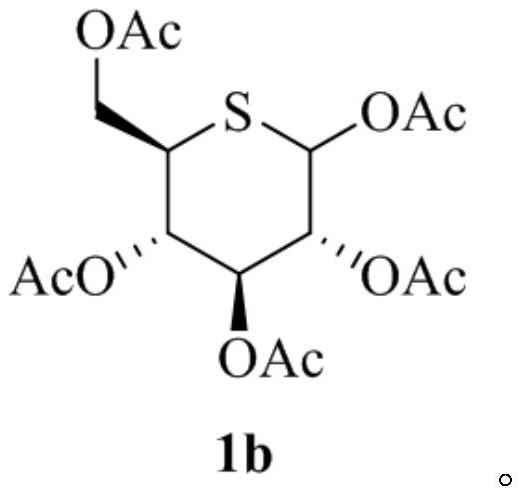
Glucosinolate compound and preparation method thereof
A technology of glucosinolates and compounds, which is applied in the field of glucosinolates and their preparation, can solve rare problems and achieve the effects of simple operation, mild reaction conditions and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] The steps for the synthesis of 5-glucosinolate in this embodiment are as follows:
[0050]
[0051]a. Compound 2 (260g, 1.0mol) was dissolved in anhydrous dichloromethane (1.5L) and anhydrous pyridine Py (2mol) was added, the solution was cooled to 0°C with ice water, and benzoyl chloride BzCl (126mL, 1.1mol), after the dropwise addition was completed, it was reacted at room temperature for 3 hours. After the reaction was completed, the reaction solution was slowly poured into a saturated solution of sodium bicarbonate at 0°C, the liquid was extracted with dichloromethane, and the extract was concentrated to obtain the oily When the product was poured into water at 0°C, a solid appeared, and the solid was washed with water for 3 times and directly reacted in the next step.
[0052] b. Dissolve the solid (compound 3) obtained in step a in 500ml of acetic acid aqueous solution (the volume fraction of acetic acid in the acetic acid aqueous solution is 70%), and stir the...
Embodiment 2
[0067] The steps for synthesizing 1,2,3,4,6-pentaacetyl 5-glucosinolate in this example are as follows:
[0068]
[0069] The steps of steps a-e are the same as in Example 1;
[0070] f. Mix the oily product (compound 7) (75g, 344mmol) obtained in step e with sodium acetate (150g), acetic anhydride (225mL) and glacial acetic acid (150ml) and heat to 140°C for reflux for 10h, then pour the reaction solution into saturated in brine, then extracted with ethyl acetate three times, the combined organic phases were washed with saturated brine, dried over anhydrous sodium sulfate, concentrated, the concentrate was dissolved in 70ml of anhydrous methanol, and the pH was adjusted to 10 with sodium methoxide, anaerobic conditions After reacting at low temperature for 2 hours, it was neutralized with cationic resin and concentrated to obtain an oily substance.
[0071] g. Dissolve the oil (compound 1a) obtained in step f in anhydrous pyridine (400ml), add acetic anhydride (244ml) and...
Embodiment 3
[0075] In this example, 5-glucosinolate and 1,2,3,4,6-pentaacetyl 5-glucose were synthesized simultaneously, and the specific steps were as follows:
[0076]
[0077] a. Dissolve compound 2 (260g, 1.0mol) in anhydrous tetrahydrofuran (1.0L) and add anhydrous pyridine Py (2.3mol), cool the solution to -2°C with ice water and add acetyl chloride AcCl (1.3mol) dropwise , after the dropwise addition was completed, react at room temperature for 4 hours. After the reaction was completed, the reaction solution was slowly poured into a saturated solution of sodium bicarbonate at 0°C, the liquid was separated and extracted with dichloromethane, the extract was concentrated, and the obtained oil was poured into Solids appeared in water at 0°C, and the solids were washed with water three times and directly reacted in the next step.
[0078] b. Dissolve the solid (compound 3) obtained in step a in 600ml of acetic acid aqueous solution (the volume fraction of acetic acid in the acetic a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com