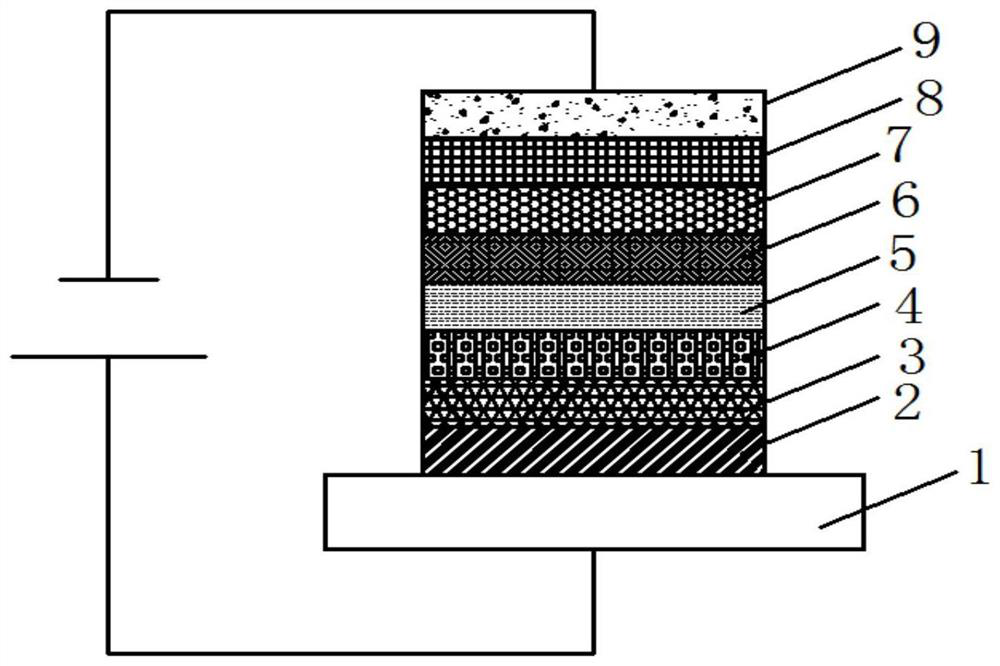
Compound containing benzophenanthrene benzofuran structure and organic electroluminescent device thereof
A compound, triphenylene technology, applied in the field of compounds containing triphenylenefuran structure and its organic electroluminescent devices, can solve the problems of backwardness and insufficient development of organic electroluminescent materials, etc., and achieve balanced transmission, good loading Flow carrier mobility, effect of reducing planarity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046]
[0047] The synthetic method of compound 1 is as follows:
[0048]
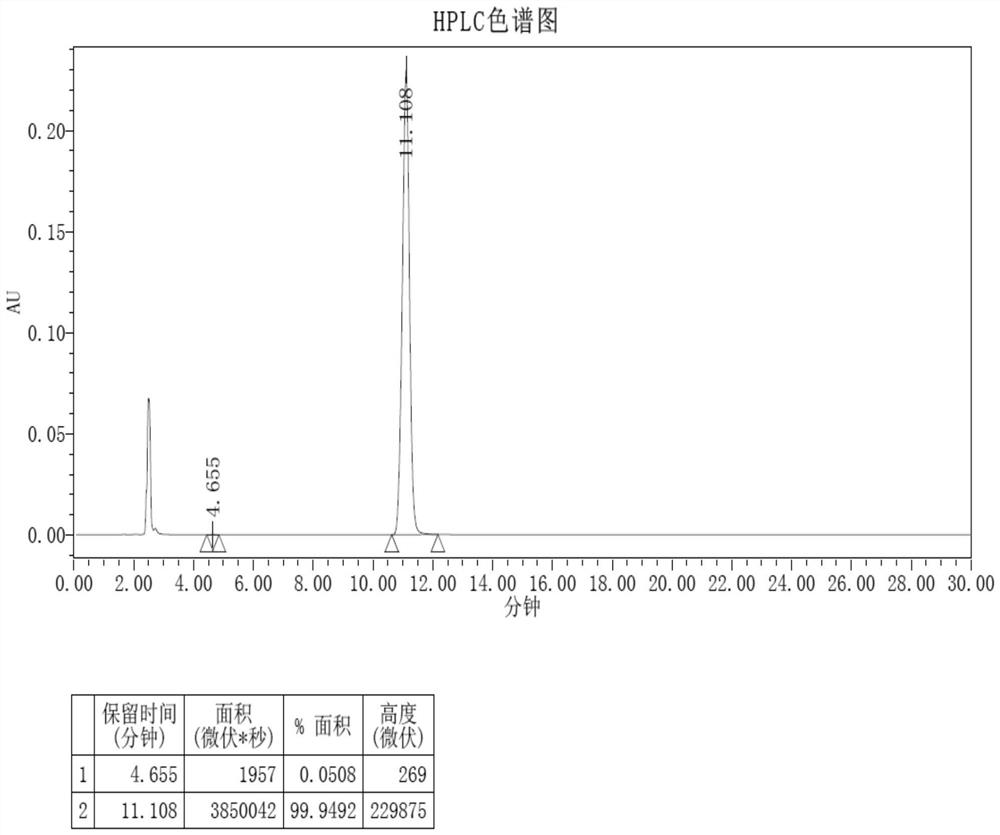
[0049] Under nitrogen protection, compound 1-a (1.1eq, 9.36g, 368.23g / mol, 25.41mmol), compound 1-b (1eq, 10g, 432.9g / mol, 23.1mmol) were dissolved in 200mL toluene, and acetic acid was added Palladium (0.26g, 224.51g / mol, 1.16mmol), X-phos (0.26g, 476.72g / mol, 1.16mmol), 100mL of ethanol and 50mL of water were added, and the reaction was stirred overnight at 100°C, and the reaction progress was monitored by HPLC.
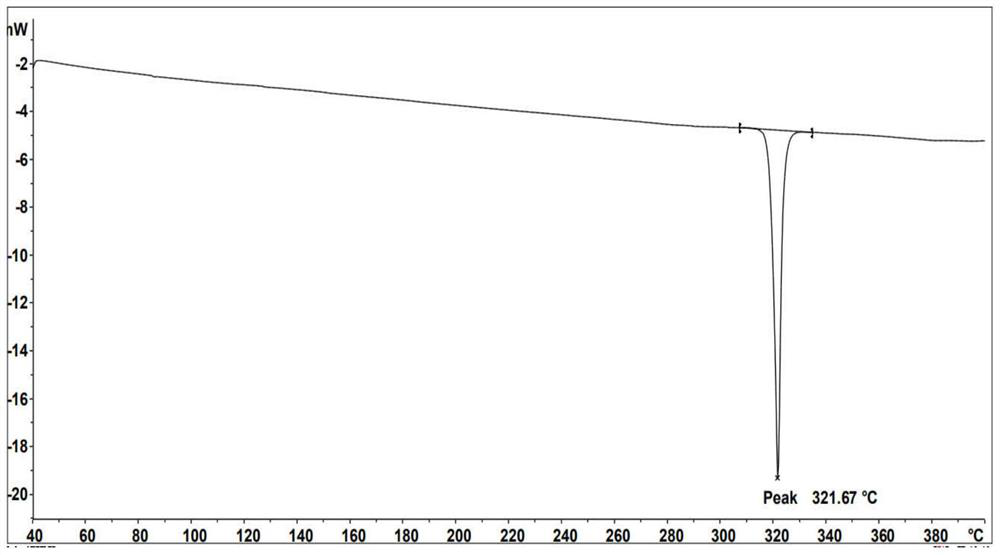
[0050] After the complete reaction of compound 1-b was monitored by HPLC, stop the reaction, cool down at room temperature, add 30 mL of water, add 1 L of ethanol, stir for 30 minutes, the solid precipitated, suction filtered, and the filter cake was collected with 80 times the weight of o-dichlorobenzene through silica gel activated carbon. Crystallized to obtain the final target product Compound 1 (8.54g, yield 57.9%), ESI-MS (m / z) (M+): theoretical value 638.71, measured value 638...
Embodiment 2
[0052]
[0053] The synthetic method of compound 4 is as follows:
[0054]
[0055]The preparation method is basically the same as in Example 1, the difference is that compound 1-b is replaced by compound 2-b to obtain the final target product compound 4 (8.46g, yield 57.5%), ESI-MS (m / z) (M+ ): theoretical value 642.74, measured value 642.52, elemental analysis results (molecular formula C45H22N4O): theoretical value C, 84.09; H, 4.70; N, 8.72; O, 2.49; measured value C, 84.01; H, 4.78; N, 8.79; O, 2.42.
Embodiment 3
[0057]
[0058] The synthetic method of compound 8 is as follows:
[0059]
[0060] The preparation method is basically the same as in Example 1, the difference is that compound 1-b is replaced by compound 3-b to obtain the final target product compound 8 (8.85g, yield 60.2%), ESI-MS (m / z) (M+ ): theoretical value 643.74, measured value 642.95, elemental analysis results (molecular formula C45H21N4O): theoretical value C, 83.96; H, 4.85; N, 8.70; O, 2.49; measured value C, 83.90; H, 4.81; N, 8.76; O, 2.53.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com