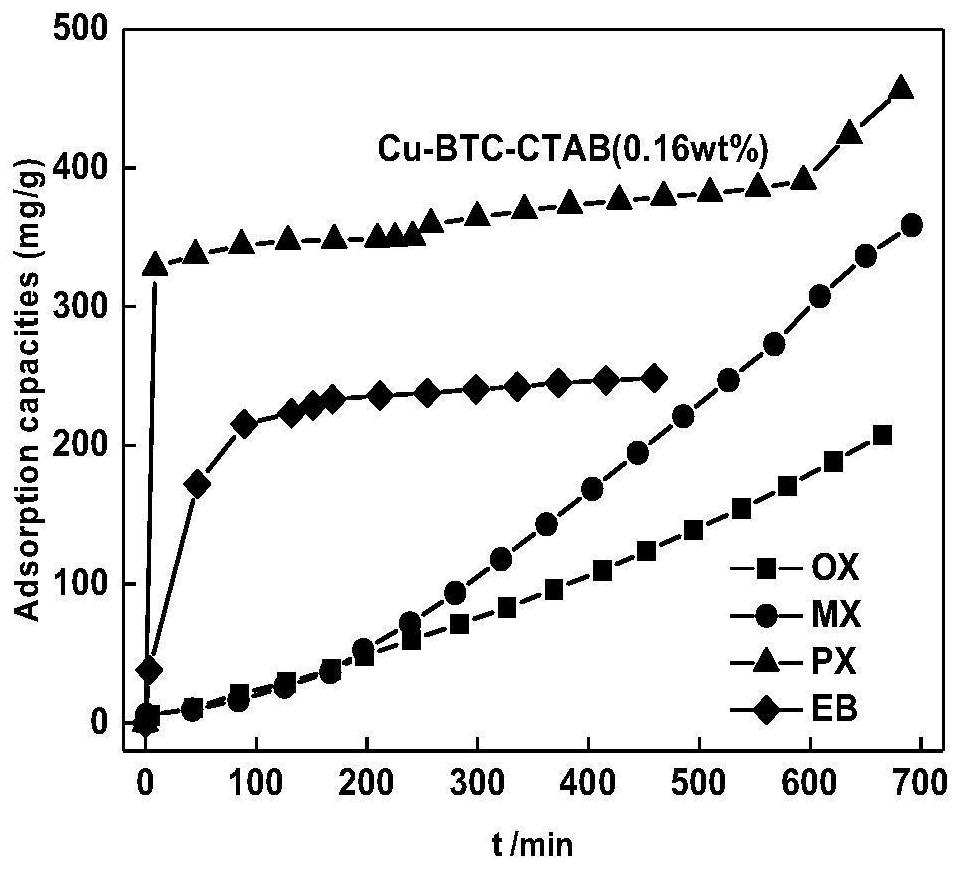
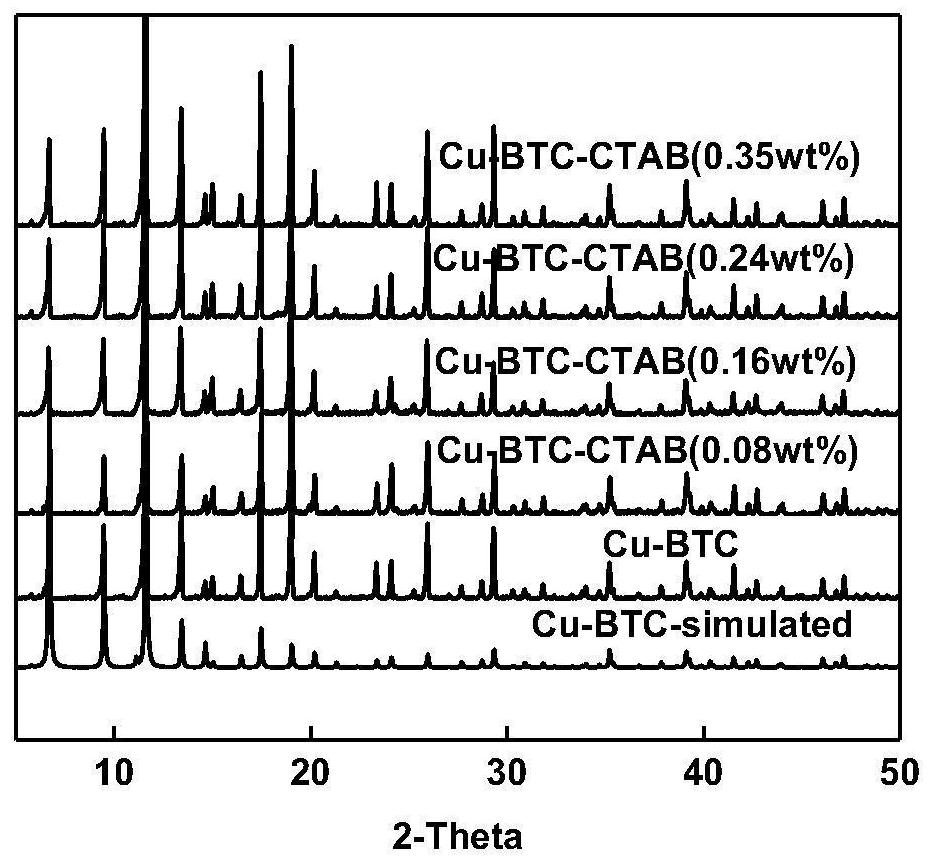
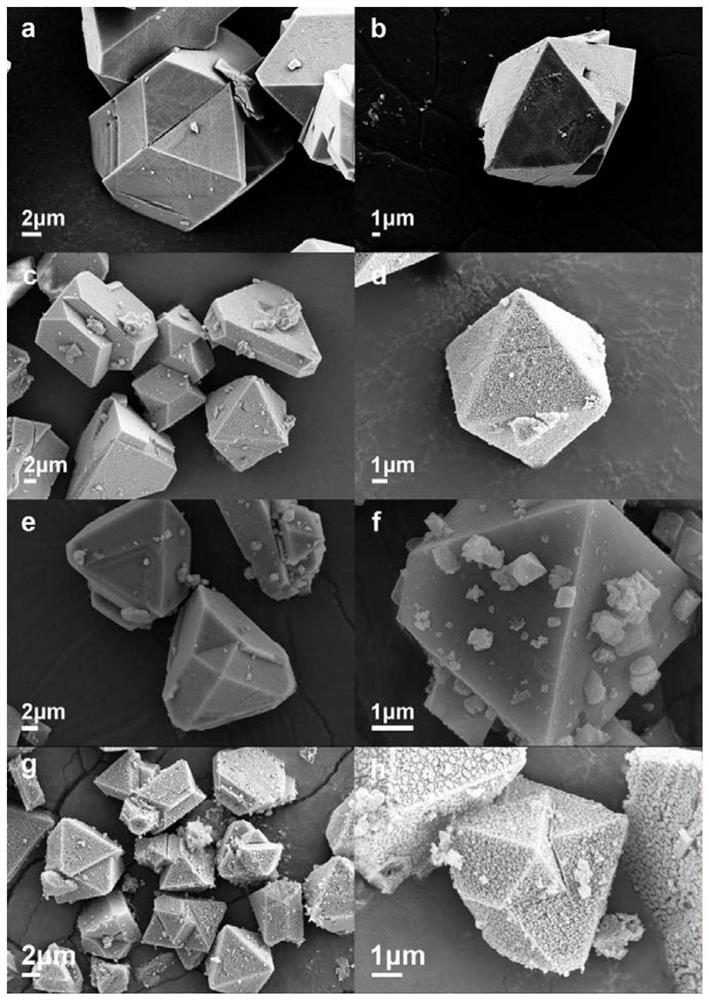
Preparation method of modified metal organic framework material for adsorbing and separating BTEX in C8 aromatic hydrocarbon
A metal-organic framework, adsorption separation technology, applied in the direction of adsorption purification/separation, organic chemistry, selective adsorption, etc., can solve the problems of low selectivity and small adsorption capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] [According to molar ratio Cu(NO 3 ) 2 ·3H 2 O:H 3 BTC:CTAB:DMSO=9:5:0.2:1130] to synthesize Cu 3 (BTC) 2 (H 2 O) 3 (CTAB):
[0030] Add H to the beaker 3 BTC (0.1051g, 0.5mmol) and DMSO 8mL, stirred and dissolved 3 ) 2 ·3H 2 O (0.2174g, 0.9mmol) was mixed and sonicated for 10min until all the solids were dissolved to form a homogeneous solution. CTAB (0.0073g, 0.02mmol) was added to the reaction kettle in advance. Transfer the homogeneous solution after ultrasound to a polytetrafluoroethylene-lined stainless steel reaction kettle, and crystallize at 120°C for 24 hours. After cooling, filter the blue-green powder at the bottom of the kettle, wash with ethanol, and put it in Dry in a drying oven at a constant temperature of 80°C for 24 hours to obtain Cu 3 (BTC) 2 (H 2 O) 3 (CTAB), the addition amount of CTAB among the embodiment 1 is 0.08wt%.
Embodiment 2
[0032] [According to molar ratio Cu(NO 3 ) 2 ·3H 2 O:H 3 BTC:CTAB:DMSO=9:5:0.4:1130] to synthesize Cu 3 (BTC) 2 (H 2 O) 3 (CTAB):
[0033]Add H to the beaker 3 BTC (0.1051g, 0.5mmol) and DMSO 8mL, stirred and dissolved 3 ) 2 ·3H 2 O (0.2174g, 0.9mmol) was mixed and sonicated for 10min until all the solids were dissolved to form a homogeneous solution. CTAB (0.01462g, 0.04mmol) was added to the reaction kettle in advance. Transfer the homogeneous solution after ultrasound to a polytetrafluoroethylene-lined stainless steel reaction kettle, and crystallize at 120°C for 24 hours. After cooling, filter the blue-green powder at the bottom of the kettle, wash with ethanol, and put it in Dry in a drying oven at a constant temperature of 80°C for 24 hours to obtain Cu 3 (BTC) 2 (H 2 O) 3 (CTAB), the CTAB addition amount is 0.16wt% among the embodiment 2.
Embodiment 3
[0035] [According to molar ratio Cu(NO 3 ) 2 ·3H 2 O:H 3 BTC:CTAB:DMSO=9:5:0.6:1130] to synthesize Cu 3 (BTC) 2 (H 2 O) 3 (CTAB):
[0036] Add H to the beaker 3 BTC (0.1051g, 0.5mmol) and DMSO 8mL, stirred and dissolved 3 ) 2 ·3H 2 O (0.2174g, 0.9mmol) was mixed and sonicated for 10min until all the solids were dissolved to form a homogeneous solution. CTAB (0.02195g, 0.06mmol) was added to the reaction kettle in advance. Transfer the homogeneous solution after ultrasound to a polytetrafluoroethylene-lined stainless steel reaction kettle, and crystallize at 120°C for 24 hours. After cooling, filter the blue-green powder at the bottom of the kettle, wash with ethanol, and put it in Dry in a drying oven at a constant temperature of 80°C for 24 hours to obtain Cu 3 (BTC) 2 (H 2 O) 3 (CTAB), the CTAB addition amount is 0.24wt% among the embodiment 3.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Saturated adsorption capacity | aaaaa | aaaaa |
| Saturated adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com