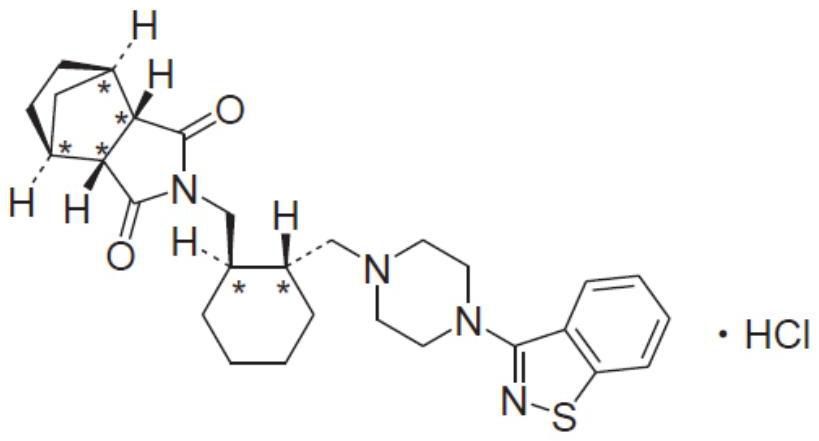
Lurasidone hydrochloride tablets and preparation method thereof
A technology of lurasidone hydrochloride tablet and lurasidone hydrochloride, which is applied in the direction of pharmaceutical formulation, pill delivery, medical preparations of non-active ingredients, etc., can solve the problem that the dissolution rate cannot be prevented, the preparation process is complicated, and the manufacturing process is increased. Cost and other issues, to achieve the effect of improving bioavailability, simple preparation process, and avoiding strict control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
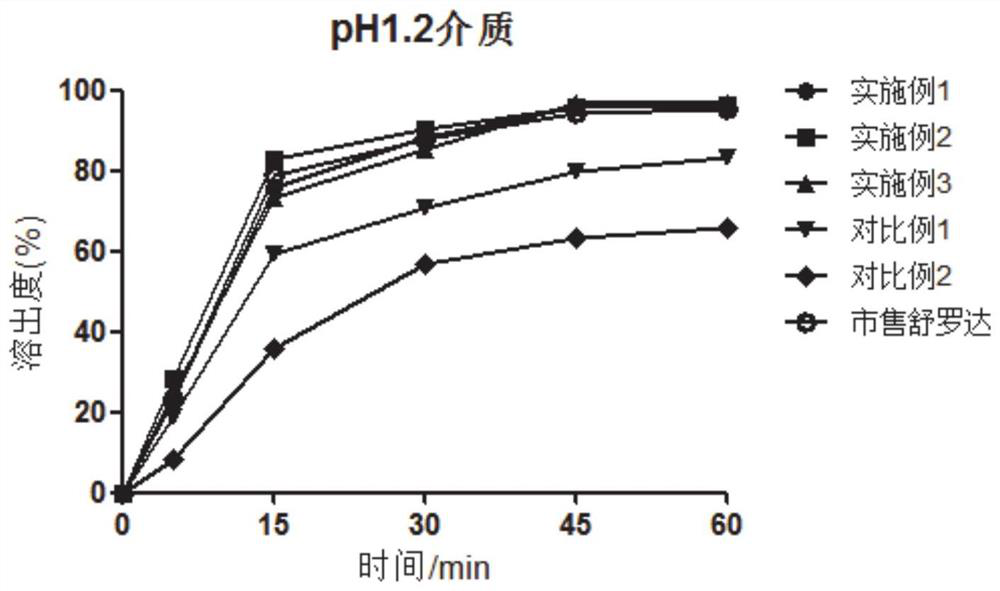
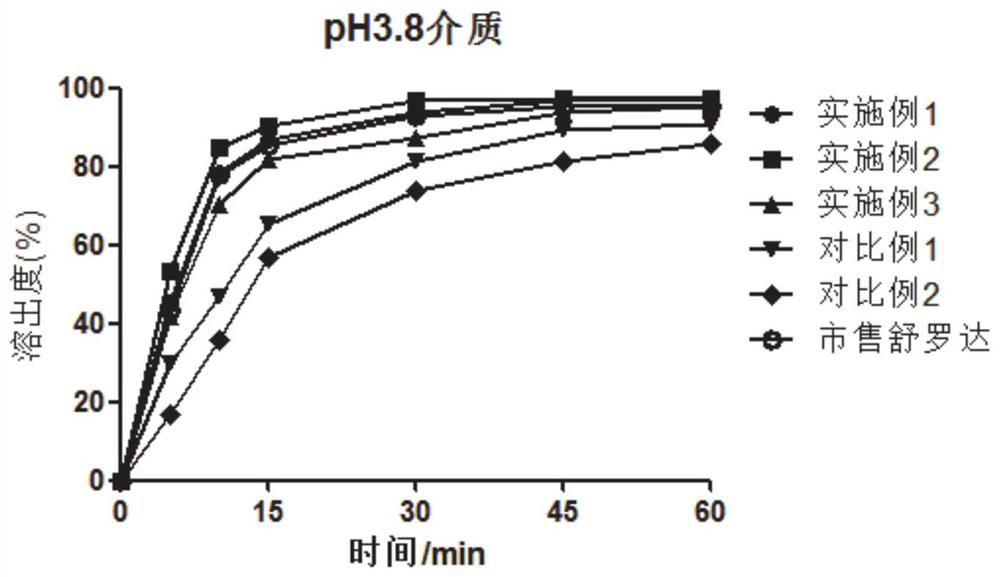
[0034] As discussed in the background technology, lurasidone hydrochloride has low solubility in the acidic environment of the stomach in the prior art, and there are many problems in the way of increasing drug dissolution in the prior art. In view of this, the present invention provides a lurasidone hydrochloride The lurasidone hydrochloride tablet has high solubility at the absorption site, high bioavailability and good stability and a preparation method thereof.
[0035] In a typical embodiment of the present invention, a lurasidone hydrochloride tablet is provided, comprising lurasidone hydrochloride, a filler, a disintegrant, a binder, and a lubricant; the binder is copovidone, Described disintegrating agent is polyvinylpolypyrrolidone; Each component is made up as follows by weight parts:
[0036] Lurasidone hydrochloride: 20-30 parts
[0037] Filling agent: 45~75 parts
[0038] Copovidone: 5-15 parts
[0039] Crospovidone: 1-15 parts
[0040] Lubricant: 0.5 to 2 par...
Embodiment 1
[0060] prescription:
[0061]
[0062]
[0063] Preparation Process:
[0064] (1) Lurasidone hydrochloride raw material medicine is pulverized with a jet mill to obtain lurasidone hydrochloride micropowder, particle size D 90 Control within the range of 15-35μm;
[0065] (2) Add lurasidone hydrochloride micropowder, filler, and crospovidone obtained in step (1) into a fluidized bed for granulation, and add a cohesive compound containing copovidone with a mass concentration of 3.0% dissolved in purified water. agent solution, the temperature of the material during granulation is controlled at 25-35°C, and after granulation is completed, dry until the loss on drying is less than 2.0%;
[0066] (3) After the granules obtained in step (2) are sized by a mobile granulator, a lubricant is added and mixed, and the resulting mixture is compressed in a rotary tablet press to obtain lurasidone hydrochloride tablets;
Embodiment 2
[0068] prescription:
[0069] composition Dosage (g / 1000 tablets) weight percentage (%) lurasidone hydrochloride 40 25.0 Mannitol 86.4 54.0 Copovidone 24 15.0 Crospovidone 8 5.0 Magnesium stearate 1.6 1.0 total 160 100
[0070] Preparation Process:
[0071] (1) Lurasidone hydrochloride raw material medicine is pulverized with a jet mill to obtain lurasidone hydrochloride micropowder, particle size D 90 Control within the range of 15-35μm;
[0072] (2) Add lurasidone hydrochloride micropowder, filler, and crospovidone obtained in step (1) into a fluidized bed for granulation, and add a cohesive compound containing copovidone with a mass concentration of 3.0% dissolved in purified water. agent solution, the temperature of the material during granulation is controlled at 25-35°C, and after granulation is completed, dry until the loss on drying is less than 2.0%;
[0073] (3) After the granules obtained in step (2) are...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com