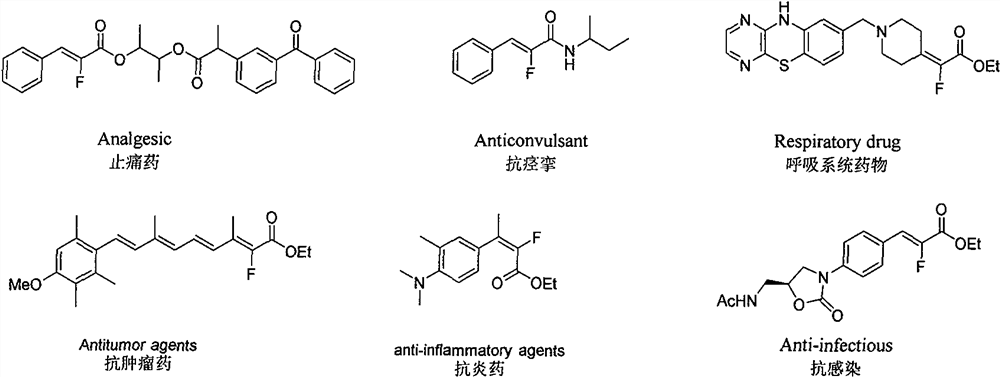
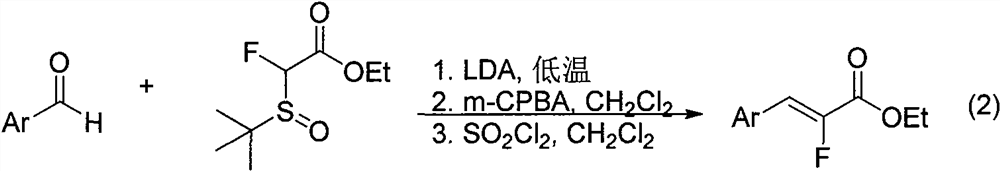
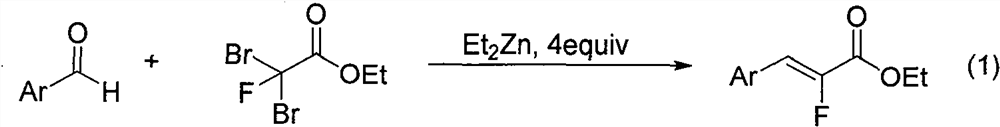Synthesis method of alpha-fluoroacrylate
A technology of fluoroacrylates and synthetic methods, which is applied in the preparation of carboxylic acid esters, chemical instruments and methods, and the preparation of carboxylic acid nitriles. It can solve the problems of harsh reaction conditions and high toxicity, and achieve short reaction time and high selectivity. , The effect of raw material economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1, the reaction formula of this embodiment is as follows:
[0038]
[0039] (1) Under air, m-bromobenzaldehyde (10mmol), diethyl fluoromalonate (1 equivalent), magnesium chloride (1 equivalent), N, N-diisopropylethylamine (DIPEA, 1 equivalent) was added Add 50 mL of anhydrous tetrahydrofuran to a 100 mL round bottom flask, and react under microwave conditions at 60°C for 30 minutes.
[0040] (2) adding ethyl acetate to the material obtained in step (1) and fully mixing, using short silica gel column chromatography, the eluent is a mixture of sherwood oil and ethyl acetate, the separation yield is 98%, and the product purity is 100%. Product Z:E = 35:1.
Embodiment 2
[0042] The reaction formula of this embodiment is as follows:
[0043]
[0044](1) Under air, m-cyanobenzaldehyde (10mmol), diethyl fluoromalonate, magnesium chloride (1 equivalent), N, N-diisopropylethylamine (DIPEA, 1 equivalent) were added as 100mL Add 50 mL of anhydrous tetrahydrofuran to a round bottom flask, and react under microwave conditions at 60°C for 30 minutes.
[0045] (2) Add ethyl acetate to the material obtained in step (1) and fully mix, use short silica gel column chromatography, the eluent is a mixture of sherwood oil and ethyl acetate, the separation yield is 97%, and the product purity is 100%. Product Z:E = 32:1.
Embodiment 3
[0047] The reaction formula of this embodiment is as follows:
[0048]
[0049] (1) Under air, perillaldehyde (10mmol), diethyl fluoromalonate (1 equivalent), magnesium chloride (1 equivalent), N, N-diisopropylethylamine (DIPEA, 1 equivalent) were added when Add 50 mL of anhydrous tetrahydrofuran to a 100 mL round bottom flask, and react under microwave conditions at 60° C. for 30 minutes.
[0050] (2) Add ethyl acetate to the material obtained in step (1) and fully mix, use short silica gel column chromatography, the eluent is a mixture of sherwood oil and ethyl acetate, the separation yield is 96%, and the product purity is 100%. Product Z:E = 30:1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com