Manganese-doped metal sulfide red fluorescent powder and preparation method thereof
A metal sulfide, red phosphor technology, applied in chemical instruments and methods, luminescent materials, etc., can solve the problems of increasing the cost of related chips, difficulty in rare earth purification, short excitation wavelength, etc., and achieve easy operation, low cost, and equipment. simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Preparation of Mn 1 Cd 6 In 28 S 52 (SH) 4 ·[H + -DBN] 8 ·[H + -PR] 4
[0032] Weigh 80 mg of indium powder, cadmium nitrate (Cd(NO 3 ) 2 4H 2 O) 40 mg, manganese acetate (Mn(Ac) 2 4H 2 (2) 5 milligrams, 120 milligrams of sulfur powders join in the polytetrafluoroethylene reactor liner, then measure piperidine (PR) 1.2 milliliters respectively, 1,8-diazabicycloundec-7-ene ( DBN) 1 ml, water 1 ml was added to the above liner. The solid-liquid mixture was stirred for 30 minutes, and then the lining of the reaction kettle was put into a stainless steel reaction kettle, sealed and heated to 190°C for 3 days. After the reaction kettle was cooled down, ethanol was added to the reaction product for multiple times of ultrasonic treatment until the solution in the upper layer of the sample was clarified, and then dried by suction filtration to obtain a crystalline manganese-doped metal sulfide red phosphor.
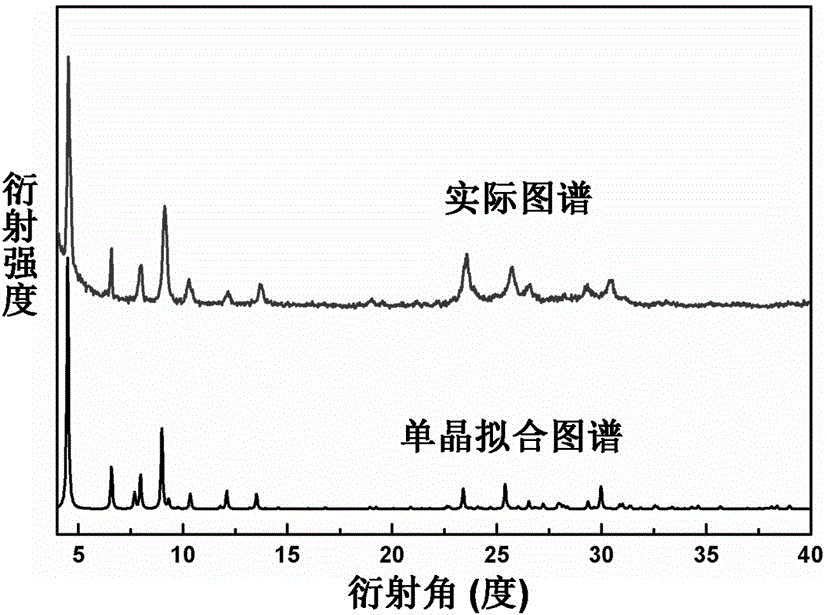
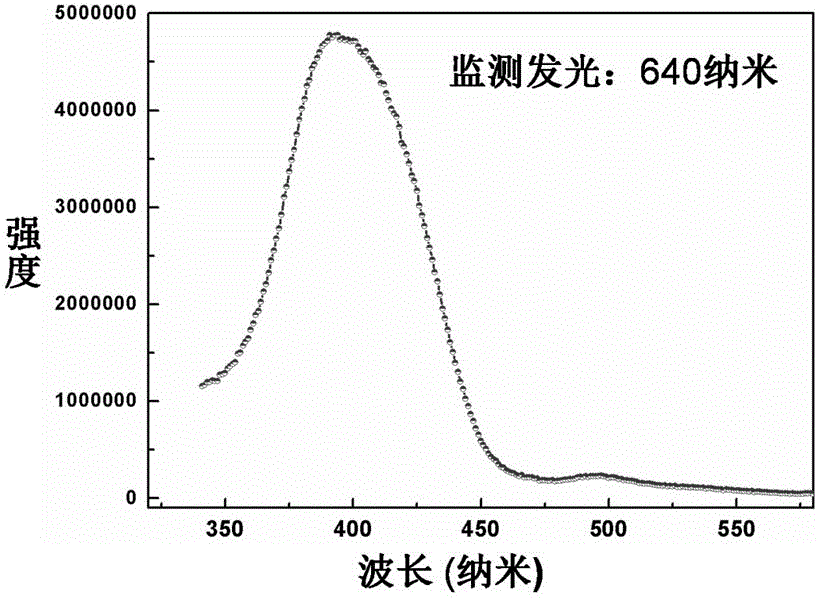
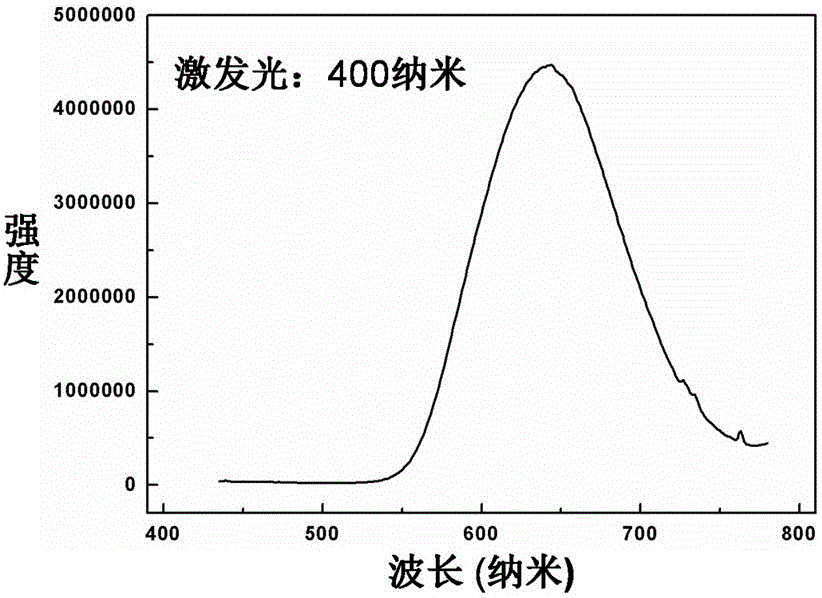
[0033] See attached figure 1 , which is the X-ray powd...
Embodiment 2
[0038] Preparation of Mn 1 Zn 6 In 28 S 52 (SH) 4 ·[H + -DBN] 8 ·[H + -PR] 4
[0039] Weigh 80 mg of indium powder, zinc acetate (Zn(Ac) 2 2H 2 O) 40 mg, manganese acetate (Mn(Ac) 2 4H 2 O) 5 milligrams, 120 milligrams of sulfur powders are added in the liner of polytetrafluoroethylene reactor, then measure respectively 1.2 milliliters of piperidine (PR), 1,8-diazabicycloundec-7-ene (DBN ) 1 ml and 1 ml of water were added to the above lining. The solid-liquid mixture was stirred for 30 minutes, and then the lining of the reaction kettle was put into a stainless steel reaction kettle, sealed and heated to 190°C for 3 days. After the reaction kettle was cooled down, ethanol was added to the reaction product, and the solution in the upper layer of the sample was sonicated several times until the solution in the upper layer of the sample was clarified, and dried by suction filtration to obtain a crystalline manganese-doped metal sulfide red phosphor.
[0040] See at...
Embodiment 3
[0045] Preparation of Mn 7 In 28 S 52 (SH) 4 ·[H + -DBN] 8 ·[H + -PR] 4
[0046] Weigh 80 mg of indium powder, manganese acetate (Mn(Ac) 2 4H 2 (O) 40 milligrams, 120 milligrams of sulfur powders were added in the polytetrafluoroethylene reactor liner, then measured respectively 1.2 milliliters of piperidine (PR), 1,8-diazabicycloundec-7-ene (DBN ) 1 ml and 1 ml of water were added to the above lining. The solid-liquid mixture was stirred for 30 minutes, and then the lining of the reaction kettle was put into a stainless steel reaction kettle, sealed and heated to 190°C for 3 days. After the reaction kettle was cooled down, ethanol was added to the inner lining of the reaction kettle, and the solution in the upper layer of the sample was sonicated several times until the solution in the upper layer of the sample was clarified, and dried by suction filtration to obtain a crystalline manganese-doped metal sulfide red phosphor.
[0047] See attached Figure 9 , which ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com