Medical EVA catheter material with surface frosted effect as well as preparation method and application of medical EVA catheter material
A catheter and frosting technology, which is applied in the field of medical polymer material modification, can solve problems such as toxicity, TPE material is not resistant to fat, and the environment is not friendly
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
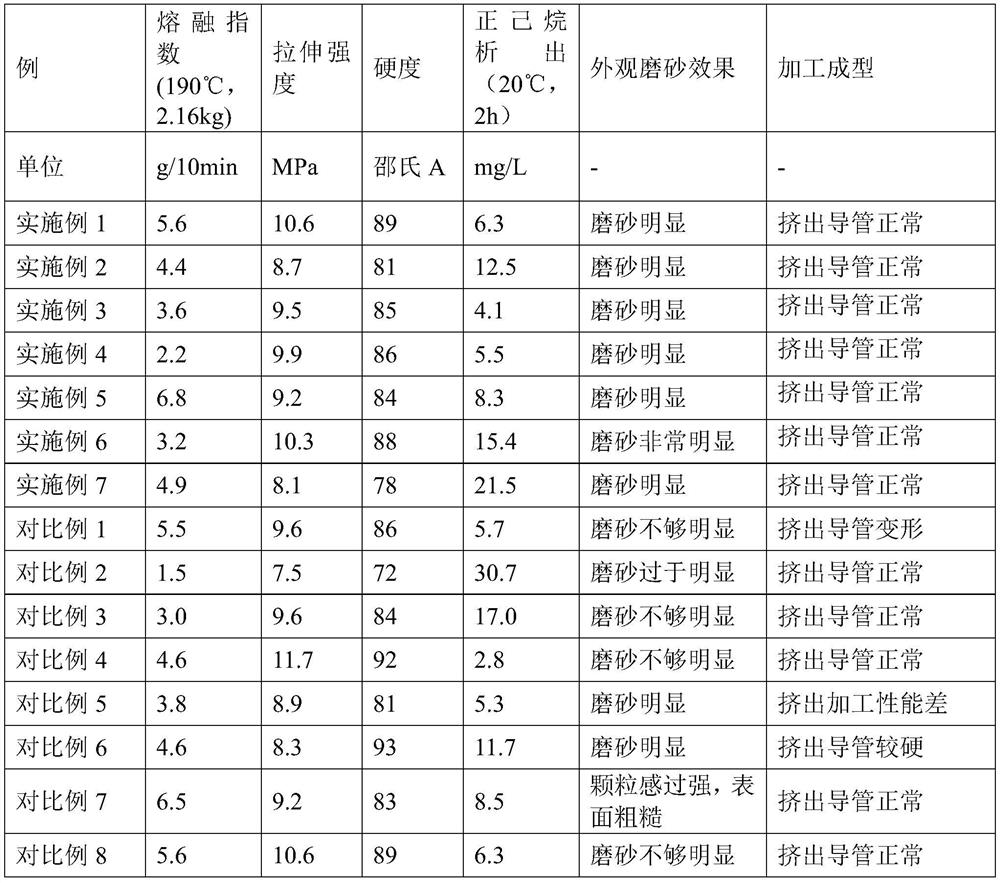
[0057] (1) Preparation of ethylene-vinyl acetate copolymer graft: 100 parts of EVA with a melt index of 7g / 10min (190°C, 2160g) were added with 0.06 parts of di-tert-butyl peroxide DTBP and 0.62 parts of EVA. Maleic anhydride MAH, after mixing uniformly, is melted and mixed by a twin-screw extruder. The temperature of the extruder is set to 160°C. After cooling and pelletizing with an underwater pelletizer, it is dehydrated and dried to obtain a melt index of 190°C and 2160g. 4.8g / 10min, graft ratio of 0.5% ethylene-vinyl acetate copolymer maleic anhydride graft.
[0058] (2) By mass, 0.2 part of antioxidant (antioxidant 1010 and antioxidant 168 = mass ratio of 1:1 are mixed), 0.5 part of slip agent (oleic acid amide, polyester graft modified Organic silicone and polytetrafluoroethylene in a ratio of 1:7:2 by mass) and 10 parts of polyethylene (melt index measured at 190°C, 2160g is 2g / 10min) weighing, high speed (800r / min) After mixing evenly, it is put into a twin-screw ext...
Embodiment 2
[0063] (1) Preparation of ethylene-vinyl acetate copolymer graft: 100 parts of EVA with a melt index of 7g / 10min (190° C., 2160g) were added with 0.08 parts of di-tert-butyl peroxide DTBP and 1.4 parts of EVA. Maleic anhydride MAH, mixed uniformly, melted and mixed by twin-screw extruder, the extruder temperature was set to 160°C, cooled and pelletized by an underwater pelletizer, and then dehydrated and dried to obtain a melt index at 190°C and 2160g. It is EVA-g-MAH with 3.5g / 10min and 1.0% grafting rate.
[0064] (2) By mass, 0.2 part of antioxidant (antioxidant 1010 and antioxidant 168 = mass ratio of 1:1 are mixed), 0.5 part of slip agent (oleic acid amide, polyester graft modified organic silicone and polytetrafluoroethylene in a mass ratio of 1:7:2) and 7 parts of propylene-based elastomer (melt index measured at 230°C, 2160g: 2.2g / 10min) Weighing at high speed (800r / min) After mixing evenly, put it into a twin-screw extruder, and obtain the auxiliary masterbatch thro...
Embodiment 3
[0069] (1) Preparation of ethylene-vinyl acetate copolymer graft: 100 parts of EVA with a melt index of 7g / 10min (190° C., 2160g) were added with 0.07 parts of di-tert-butyl peroxide DTBP and 1.1 parts of EVA. Maleic anhydride was mixed uniformly and then melted and mixed by a twin-screw extruder. The temperature of the extruder was set at 160°C. After cooling and pelletizing with an underwater pelletizer, the product was dehydrated and dried to obtain a melt index of 190°C and 2160g. 4.3g / 10min, EVA-g-MAH with a grafting rate of 0.8%.
[0070] (2) By mass, 0.2 part of antioxidant (antioxidant 1010 and antioxidant 168 = mass ratio of 1:1 are mixed), 0.5 part of slip agent (oleic acid amide, polyester graft modified silicone and polytetrafluoroethylene in a mass ratio of 1:7:2) and 3 parts of vinyl elastomer (density 0.908g / cm 3 , measured at 190°C and 2160g with a melt index of 1g / 10min), weighed at high speed (800r / min) and mixed evenly, put it into a twin-screw extruder, an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melt index | aaaaa | aaaaa |
| Melt index | aaaaa | aaaaa |
| Melt index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com