Antidote composition and application thereof
A composition and drug technology, applied in the field of preparation of poisoning prevention and treatment drugs, can solve the problems of lack of organ protection, only focusing on removal of poisons and detoxification, poor prognosis of patients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Therapeutic effect on rats with acute organophosphate (dichlorvos) poisoning
[0032]Effects on the effect of non-lethal dose of dichlorvos: 72 healthy rats, half male and half male, were randomly divided into 6 groups according to sex and weight, 12 rats in each group, respectively normal control group, exposure group (30mg / kg dichlorvos), Drug exposure (30mg / kg dichlorvos) + levocarnitine 600mg / kg gavage treatment group, drug exposure (30mg / kg dichlorvos) + levocarnitine 600mg / kg intraperitoneal injection treatment group, drug exposure (30mg / kg dichlorvos) + pralidoxime chloride 30mg / kg injection treatment group, poison exposure (30mg / kg dichlorvos) + levocarnitine 600mg / kg injection + pradoxime chloride 30mg / kg injection treatment group. Except for the normal group, after fasting for 24 hours, the animals were given 30 mg / kg of dichlorvos by intragastric administration once, and the intragastric volume was 1 ml / 100 g. Corresponding drug treatment was give...
Embodiment 2
[0045] Embodiment 2: Therapeutic effect on metal (lead) poisoning mice
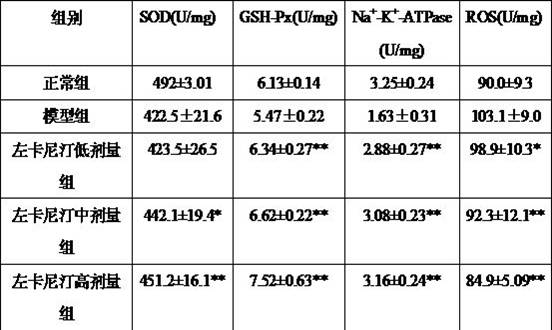
[0046] Seventy-two healthy and clean Kunming male mice born one week old, with a body weight of 10±2 grams, were randomly divided into 6 groups after adaptive feeding for 1 week, lead acetate model group, edetate disodium calcium group ( 75mg / kg, i.m), levocarnitine 100mg / kg, 200mg / kg, 400mg / kg group, edetate disodium calcium + levocarnitine 400mg / kg group, 12 rats in each group. The mice in the normal control group were given 0.4ml of deionized water, and the other groups were given 0.4ml of deionized water containing lead acetate (lead ion content 2mg / L). drug intervention. Continuous treatment for 14 days, 24 hours after the last intervention, blood was taken from the orbit, anticoagulated with heparin sodium, mixed with 4:1 (volume fraction) concentrated nitric acid / perchloric acid solution, heated and digested. Killed by neck dissection, quickly separated the right femur, removed its peripheral fat...
Embodiment 3
[0051] Embodiment 3: to the therapeutic effect of acute carbon monoxide poisoning rat
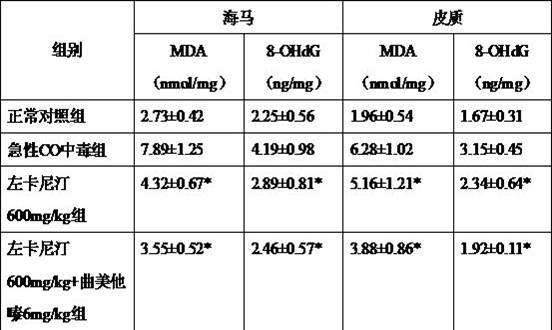
[0052] Acute carbon monoxide poisoning model establishment and drug intervention: 60 male SD rats, body weight 220-250 g, were divided into normal control group, acute CO poisoning group, levocarnitine 600mg / kg group, levocarnitine 600mg / kg+ In the trimetazidine 6 mg / kg group, there were 12 rats in the normal control group and 16 rats in the other groups. According to the method reported in the literature, the rats were placed in the exposure box, continuously inhaled CO with a concentration of 2 000 ppm for 35 min, and then continuously inhaled CO with a concentration of 3 000 ppm for 25 min before leaving the box; Air was continuously inhaled in the poison box for 1 h. Immediately after the exposure, the modified Rodkey FL microquantitative method was used to detect the blood carboxyhemoglobin concentration (COHb%). When COHb% > 40%, the model was considered successful. Rats in the trea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com