A kind of preparation method of improved sacillin hydroiodide
A technology of sacillin hydroiodide and sacillin citrate, which is applied in the field of preparation of sacillin hydroiodide, can solve the problems of poor product stability, storage of unfavorable product purification intermediates and control of impurities, etc. , to achieve the effects of easy storage and transportation, easy separation and purification, and safe production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
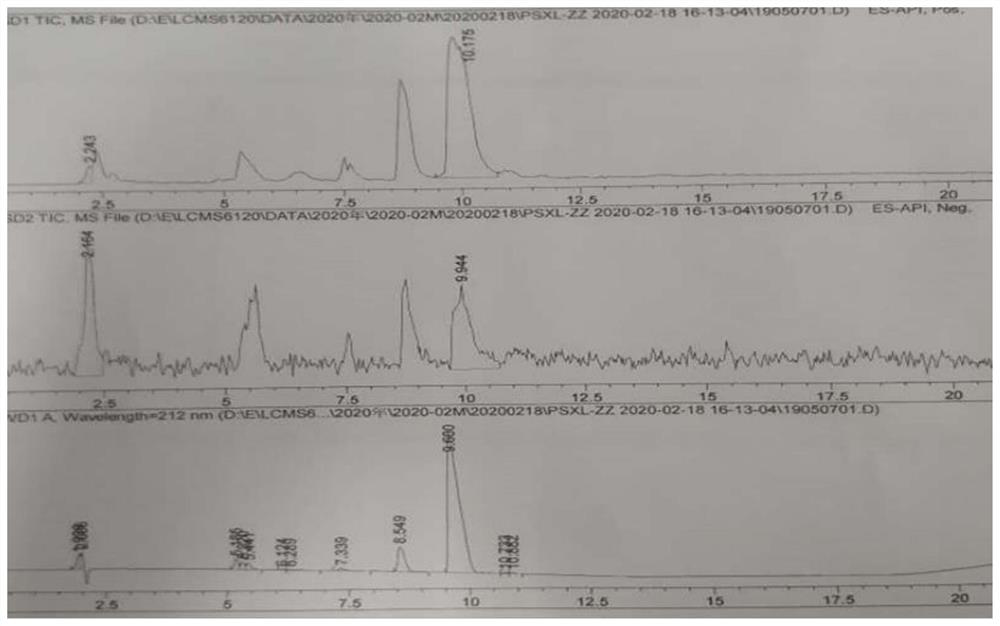
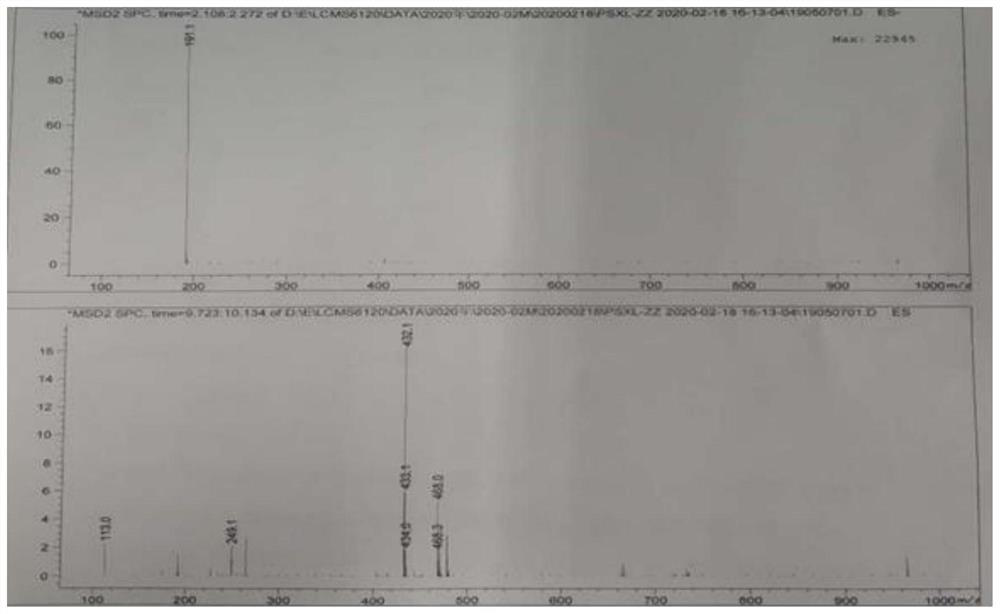
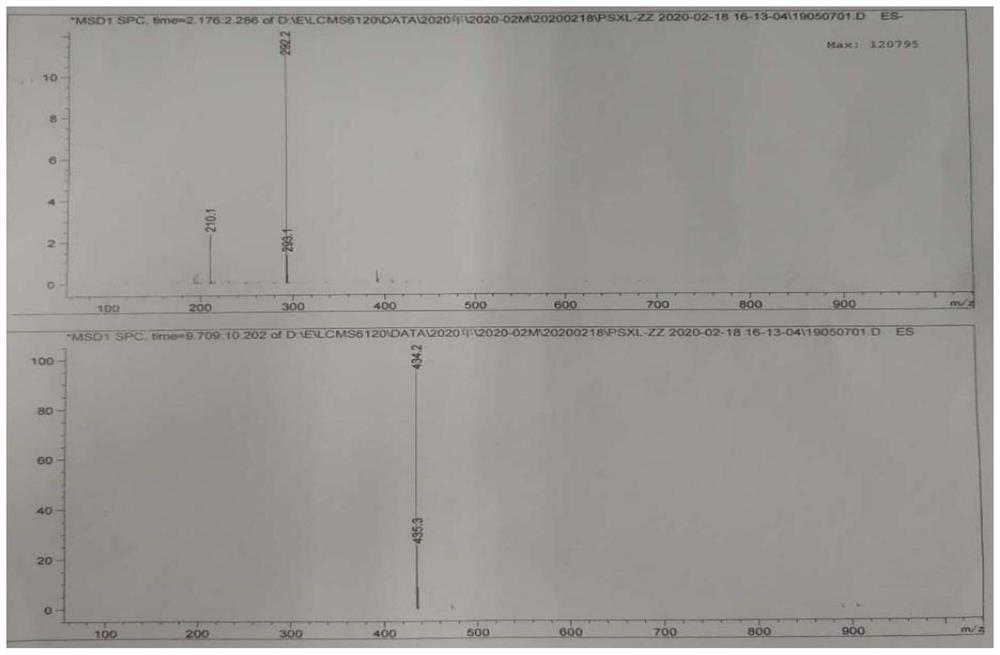
Image
Examples
Embodiment 1
[0049] In a 250ml beaker, add 40.0g water, cool to 10°C, add 25.0g β-chlorotriethylamine hydrochloride, stir to dissolve; take another 250ml beaker, add 37.5g water and 21.0g potassium carbonate, stir until complete Dissolve; drop the prepared potassium carbonate solution into the chlorotriethylamine hydrochloride solution, heat up to 20° C., let stand for 1 hour, and separate the layers. The upper organic phase is β-chlorotriethylamine.
[0050] Take a 500ml three-necked flask, add the above-mentioned organic phase, 200.0g of acetone, and 40.0g of penicillin potassium, stir evenly, heat up and reflux, and react for 4 hours; filter to remove impurities, wash the filter cake with 80.0g of acetone, combine the organic phases, and add 22.38g of citric acid , grow the crystals for 2 hours; filter, wash the filter cake with 12.36g acetone, suck dry, and after the solid is dried at 40°C, 66.61g of sacillin citrate can be obtained. The yield was 99.1%, and the purity was 99.95% by HP...
Embodiment 2
[0054] Take a 1000ml beaker, add 50.0g of sacillin citrate, stir and dissolve with 550g of water at 40°C, filter into a three-necked bottle for later use; dissolve 18g of sodium iodide dihydrate in 25g of water, filter and set aside;
[0055] The temperature in the temperature-controlled three-necked flask was 0°C, the stirring speed was 200 rpm, the sodium iodide solution was slowly added to the citrate system, the addition was completed, and the crystal was grown for 2 hours; filtered, washed with 50 g of water, drained, and the solid Dry to constant weight (7 hours) at 40° C. to obtain 40.92 g of pentacillin hydroiodide. The yield is 90.4%, and the HPLC detection and analysis show that the purity is 99.86%, and the maximum single impurity is less than 0.1%.
Embodiment 3
[0057] Take a 1000ml beaker, add 550g of water, heat it up to 20°C, add 50.0g of pisacillin citrate, stir to dissolve, filter into a three-necked bottle for later use; dissolve 20g of sodium iodide dihydrate in 28g of purified water, filter, and wait for use;
[0058] The temperature in the temperature-controlled three-necked flask was 3°C, the stirring speed was 200 rpm, the sodium iodide solution was slowly added to the citrate system, the addition was completed, and the crystal was grown for 1.5 hours; filtered, washed with 40 g of water, drained, and the solid After drying at 20° C. to constant weight (7 hours), 40.12 g of pentacillin hydroiodide was obtained. The yield was 88.9%, and the HPLC detection and analysis showed that the purity was 99.75%, and the maximum single impurity was less than 0.1%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com