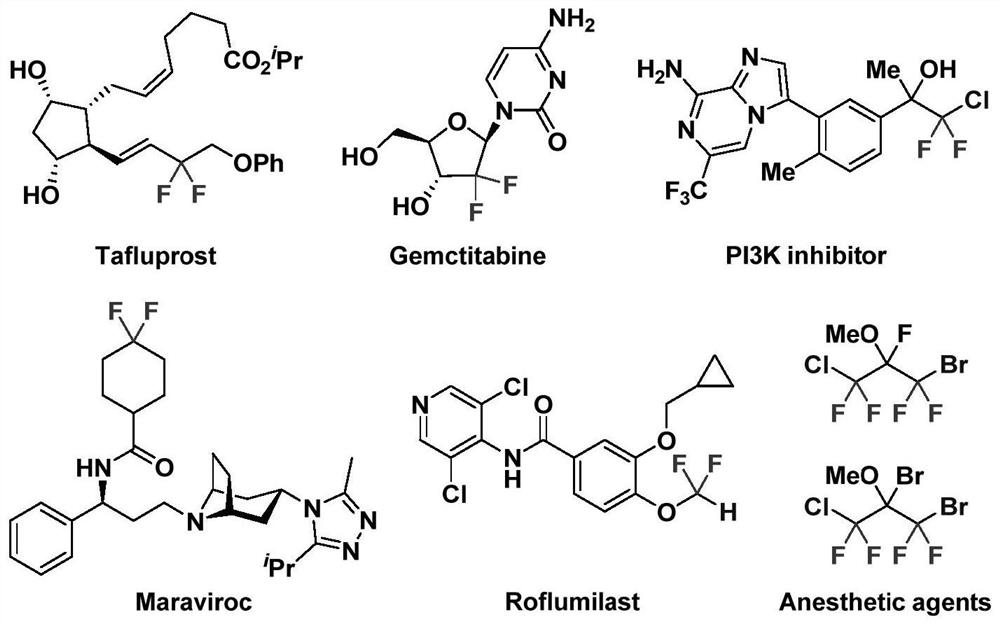
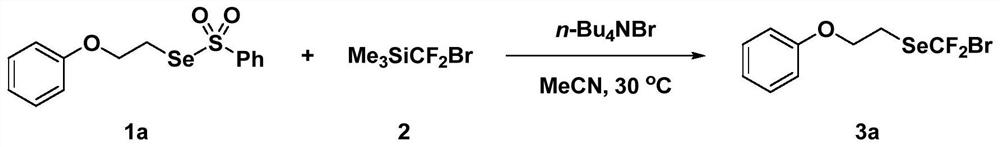
Method for synthesizing bromodifluoromethyl selenide compound under metal-free condition
A technology of bromodifluoromethylselenide and difluoromethylselenide, which is applied in the field of chemical synthesis, can solve the problems of unfavorable large-scale popularization and application, narrow substrate range, complicated operation, etc., and achieve large-scale production and application, The effect of wide substrate universality and reduced operation difficulty
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Embodiment 1: the preparation of (bromodifluoromethyl) (2-phenoxyethyl) selenide
[0059] This embodiment provides the preparation method of (bromodifluoromethyl) (2-phenoxyethyl) selenide, and the specific synthesis steps are:
[0060] Take a clean 8mL screw-top reaction bottle, add a 5*10mm magnetic stirrer, take Se-(2-phenoxyethyl)selenobenzenesulfonate (1a, 136.5mg, 0.4mmol) and tetrabutyl bromide Ammonium (64.5 mg, 0.2 mmol) was dissolved in 2 mL of acetonitrile. Then (bromodifluoromethyl)trimethylsilane (2, 98 μL, 0.60 mmol) was added, the bottle was capped, stirred in an oil bath at 30° C. (500 rpm), and reacted for 30 minutes. After the reaction was over, the reaction system was transferred to an eggplant-shaped flask, and 800 mg of column chromatography silica gel powder was added, and the solvent was removed by rotary evaporation, and then the column chromatography was separated with sherwood oil as an eluting agent to obtain the target product (bromodifluoro...
Embodiment 2
[0066] Embodiment 2: the amplified synthesis of (bromodifluoromethyl) (2-phenoxyethyl) selenide
[0067] This embodiment provides a large-scale synthetic method of (bromodifluoromethyl) (2-phenoxyethyl) selenoether, and the specific synthetic steps are:
[0068] Take a clean 50mL round-bottomed reaction bottle, add a 20*10mm magnetic stirrer, take Se-(2-phenoxyethyl)selenobenzenesulfonate (1a, 1.365g, 4.0mmol) and tetrabutyl bromide Ammonium (0.645 g, 2.0 mmol) was dissolved in 20 mL of acetonitrile. Then (bromodifluoromethyl)trimethylsilane (2, 0.98 mL, 6.0 mmol) was added, and the bottle was capped, stirred in an oil bath at 30° C. (500 rpm), and reacted for 60 minutes. After the reaction finished, the reaction system was transferred to an eggplant-shaped flask, 2.0g column chromatography silica gel powder was added, the solvent was removed by rotary evaporation, and then the column chromatography separation was carried out with sherwood oil as a eluting agent to obtain the...
Embodiment 3
[0070] Embodiment 3: Preparation of (bromodifluoromethyl) (2-(4-methoxyphenoxy) ethyl) selenoether
[0071] This embodiment provides the preparation method of (bromodifluoromethyl)(2-(4-methoxyphenoxy)ethyl)selenoether, the specific synthesis steps are:
[0072] Take a clean 8mL screw-top reaction bottle, add a 5*10mm magnetic stir bar, and take Se-(2-(4-methoxyphenoxy)ethyl)selenobenzenesulfonate (1b, 148.5mg, 0.4mmol ) and tetrabutylammonium bromide (64.5 mg, 0.2 mmol) were dissolved in 2 mL of acetonitrile. Then (bromodifluoromethyl)trimethylsilane (2, 98 μL, 0.60 mmol) was added, the bottle was capped, stirred in an oil bath at 30° C. (500 rpm), and reacted for 30 minutes. After the reaction was over, the reaction system was transferred to an eggplant-shaped flask, and 800 mg of silica gel powder for column chromatography was added. / v) As eluent, perform column chromatography separation to obtain the target product (bromodifluoromethyl)(2-(4-methoxyphenoxy)ethyl)selenid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com