Synthesis method of 2-fluoro-5-trifluoromethylpyrimidine
A technology of trifluoromethylpyrimidine and synthesis method, which is applied in the field of drug synthesis, and can solve the problems of high cost of raw materials, complicated process, and high cost of antimony pentafluoride
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] The invention provides a kind of synthetic method of 2-fluoro-5-trifluoromethylpyrimidine, comprising the following steps:
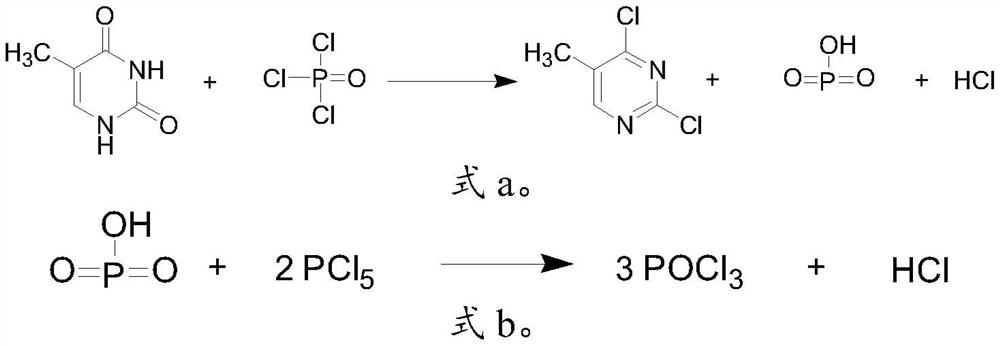
[0030] Mix 5-methyluracil, phosphorus oxychloride and phosphorus pentachloride for ring chlorination to obtain 2,4-dichloro-5-methylpyrimidine;
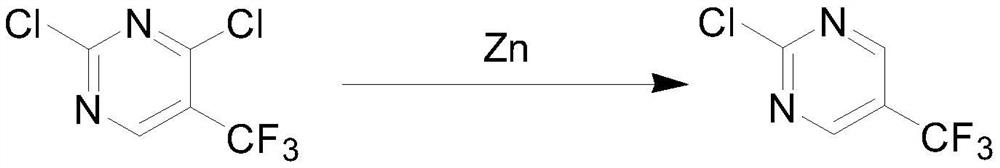
[0031] Mixing the 2,4-dichloro-5-methylpyrimidine and zinc powder for reduction reaction to obtain 2-chloro-5-methylpyrimidine;
[0032] The 2-chloro-5-methylpyrimidine is mixed with the initiator, and chlorine gas is introduced to perform a chlorination reaction to obtain 2-chloro-5-trichloromethylpyrimidine;
[0033] The 2-chloro-5-trichloromethylpyrimidine and hydrogen fluoride are mixed for fluorination reaction to obtain 2-fluoro-5-trifluoromethylpyrimidine.
[0034] In the invention, 5-methyluracil, phosphorus oxychloride and phosphorus pentachloride are mixed for ring chlorination reaction to obtain 2,4-dichloro-5-methylpyrimidine. In the present invention, the molar ratio of said 5-methyluracil...
Embodiment 1
[0057] Take 75g (0.59mol) of 5-methyluracil, 236g of phosphorus oxychloride, and 16.5g (0.12mol) of triethylamine hydrochloride, add them into the reaction flask, raise the temperature to 100°C to 110°C, and reflux for 5h. Cool down to 40°C, add phosphorus pentachloride 248 (1.19mol), and keep the reaction for 2h. After the reaction was completed, phosphorus oxychloride was recovered by vacuum distillation, and the vacuum distillation was continued to obtain 88 g (0.54 mol) of 2,4-dichloro-5-methylpyrimidine, with a yield of 91.5%.
Embodiment 2
[0059] Take 70g (0.43mol) of 2,4-dichloro-5-methylpyrimidine obtained in Example 1, 84g (1.28mol) of zinc powder, and 700g of water, put them into the reaction bottle, and reflux at 95°C to 105°C for 5h , after the reaction was completed, the product material liquid was extracted with dichloromethane, and the dichloromethane phase was distilled to reclaim the dichloromethane, and the crude product obtained was recrystallized with petroleum ether to obtain 45 g of 2-chloro-5-methylpyrimidine (0.35 mol ), yield 81.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com