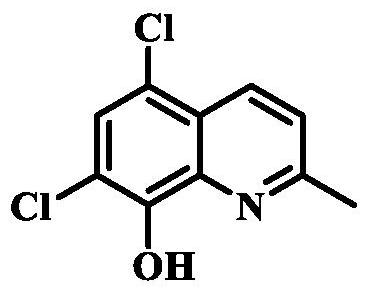
Preparation method of chlorquinaldol
A technology of chloroquinadol and methyl quinoline, applied in the field of drug synthesis, can solve the problems of large amount of sodium hypochlorite solution, increase the cost of treating waste liquid, reduce yield and the like, meet the requirements of reaction equipment, and reduce waste liquid. Generate, improve quality and yield effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Put 10g of 8-hydroxy-2-methylquinoline, 50mL of dichloromethane and 0.35g of aluminum chloride into a 250mL reaction bottle, stir and cool down to 20~30℃, add 14.2g of tert-butyl hypochlorite, 32~41 ℃ insulation reaction for 7h.
[0068] Cool the reaction solution to 20-30°C, filter, add 15mL of concentrated hydrochloric acid dropwise into the filtrate, precipitate out, filter, add 150mL of water, stir to dissolve, slowly add ammonia water until the pH is 3.0, precipitate solid, filter, rinse, Dried rough.
[0069] The crude product was refined with 160 mL of absolute ethanol and 20 mL of water to obtain 9.1 g of pure chloroquinaldol, with a yield of 63.28% and a HPLC purity of 99.58%.
Embodiment 2
[0071] Put 20g of 8-hydroxy-2-methylquinoline, 200mL of chloroform and 1.0g of aluminum chloride into a 500mL reaction bottle, stir and cool down to 20-30°C, add 28.0g of tert-butyl hypochlorite, and keep warm at 32-41°C Reaction 8h.
[0072] Cool the reaction solution to 20-30°C, filter, add 20mL of concentrated hydrochloric acid dropwise to the filtrate, precipitate out, filter, add 200mL of water, stir to dissolve, slowly add ammonia water until the pH is 3.5, precipitate solid, filter, rinse, Dried rough.
[0073] The crude product was refined with 280 mL of absolute ethanol and 30 mL of water to obtain 19.7 g of pure chloroquinaldol, with a yield of 68.86% and a HPLC purity of 99.65%.
Embodiment 3
[0075] Put 10g of 8-hydroxy-2-methylquinoline, 80mL of dichloromethane and 0.5g of aluminum chloride into a 250mL reaction bottle, stir and cool down to 20-30°C, add 14g of tert-butyl hypochlorite, 32-41°C Insulation reaction 9h.
[0076] Cool the reaction solution to 20-30°C, filter, add 12mL of concentrated hydrochloric acid dropwise into the filtrate, precipitate out, filter, add 100mL of water, stir to dissolve, slowly add ammonia water until the pH is 4.0, precipitate solid, filter, rinse, Dried rough.
[0077] The crude product was refined with 100 mL of acetonitrile and 10 mL of water to obtain 9.5 g of pure chloroquinaldol, with a yield of 66.05% and an HPLC purity of 99.70%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com