Cat granulocyte colony stimulating factor mutant recombinant fusion protein as well as preparation method and application thereof
A technology of colony stimulating factor and fusion protein, which is applied in the field of biological genetic engineering to achieve the effects of reducing preparation cost, improving protein stability and enhancing biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Embodiment 1 Preparation of recombinant FeG-CSF-Mut / RTBD1 fusion protein
[0058] 1. Construction of expression vector for feline granulocyte colony-stimulating factor (FeG-CSF) mutation
[0059] (1) Carry out homologous modeling of feline granulocyte colony-stimulating factor molecule and feline granulocyte colony-stimulating factor receptor molecule through the SWISS-MODEL online platform; The colony-stimulating factor receptor molecule was docked to obtain the complex structure model; the full-length sequence of the complex structure model was mutated by alanine scanning to obtain potential mutation sites; the mutation was calculated for a single mutation site by virtual saturation mutation Changes in intermolecular binding force before and after.
[0060] (2) Preparation of recombinant plasmid pUC57-FeG-CSF:
[0061] The feline granulocyte colony-stimulating factor gene sequence was queried through the NCBI Genbank database, and the obtained sequence was subjected...
Embodiment 2
[0082] Embodiment 2 Pharmacodynamics test of recombinant FeG-CSF-Mut / RTBD1 fusion protein
[0083] 1. Construction of leukopenia mouse model
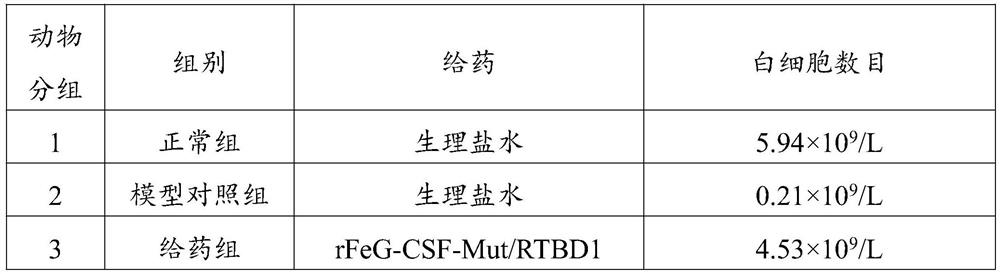
[0084] Select 18 mice and intraperitoneally inject cyclophosphamide 2 mg / mouse·day for three consecutive days. On the fourth day, the whole blood of the mice was taken for blood routine, and the number of white blood cells (WBC) was detected. The success standard of the model used in this experiment was: the number of white blood cells was lower than the lowest value of the reference range by 0.8×10 9 / L. Mice that meet the above conditions are deemed to have successfully prepared the mouse model of leukopenia. The normal group and the model control group were subcutaneously administered with normal saline on the second day after modeling, and the administration group was subcutaneously administered 0.2 μgrFeG-CSF-Mut / RTBD1 fusion protein to the back of the neck of the mice on the second day after modeling.
[0085] Table 2 Grouping ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com